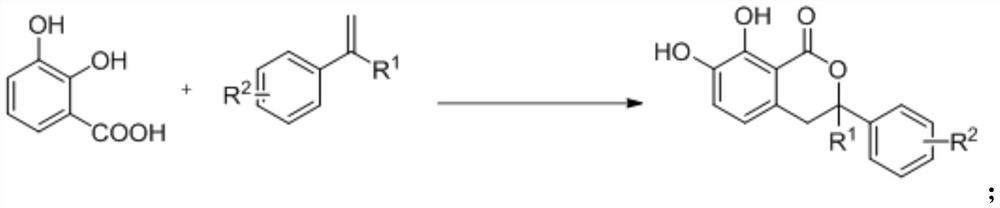
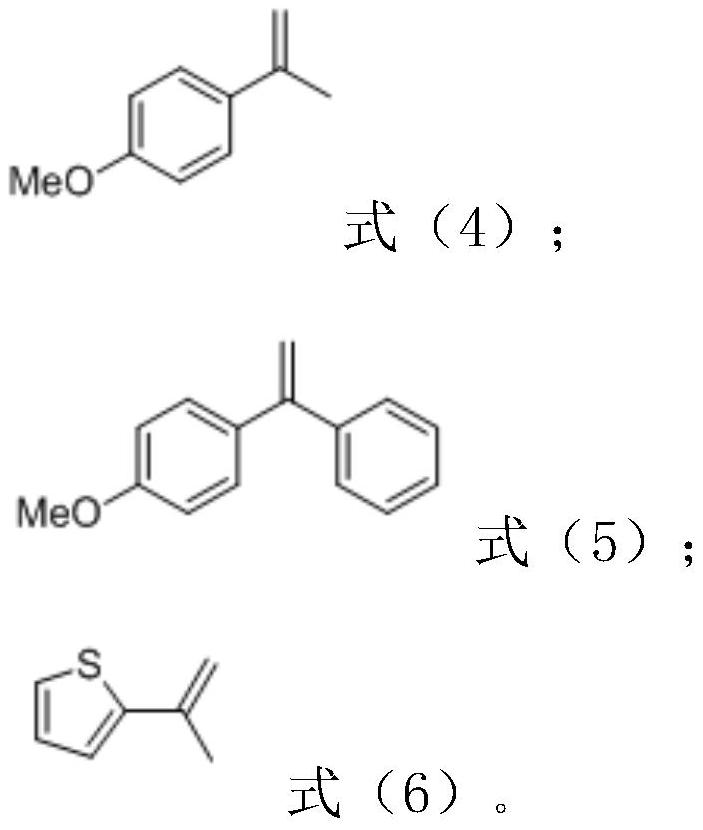
Isochroman derivative and enzymatic synthesis method and application thereof
A synthetic method, the technology of isochroman, which is applied in the field of isochroman derivatives and their enzyme-catalyzed synthesis, can solve the problems of complicated operation and heavy pollution, and achieve the effects of simple operation, improved conversion rate and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
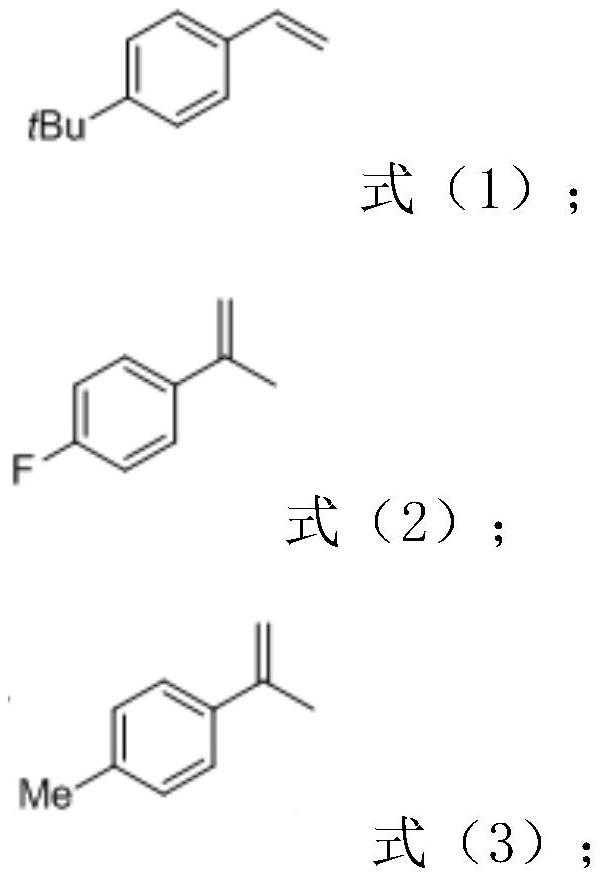
[0033] Add 2,3-dihydroxybenzoic acid (0.3mmol, 46.2mg), 4-tert-butylstyrene (0.15mmol, 27.5uL), MnxEFG (0.01mol%), hydrogen peroxide to a 10ml reaction flask in sequence (0.75mmol, 75uL), deionized water (1mL), acetonitrile (1mL), hydrochloric acid (0.3mmol, 13uL) magnetically stirred at room temperature for 48 hours, after the conversion of the substrate, ethyl acetate was added to the reaction system to extract (3mL *4), the organic phases were combined and dried with anhydrous sodium sulfate, filtered and concentrated in vacuo to obtain a crude product. The obtained crude product was purified and separated by column chromatography to obtain a white solid product with an isolated yield of 77%.
[0034] The synthetic route of embodiment 1 is as follows:
[0035]
[0036] The proton nuclear magnetic resonance spectrum of the target product obtained in Example 1 is as follows.
[0037] 1 H NMR (400MHz, CDCl 3 ):11.06(s,1H),7.91–7.77(m,1H),7.46(d,J=8.4Hz,2H),7.41(d,J=8.4H...
Embodiment 2
[0040] Add 2,3-dihydroxybenzoic acid (0.3mmol, 46.2mg), 4-fluoro-α-methylstyrene (0.15mmol, 20.2uL), MnxEFG (0.01mol% ), hydrogen peroxide (0.75mmol, 75uL), deionized water (1mL), acetonitrile (1mL), hydrochloric acid (0.3mmol, 13uL) were magnetically stirred at room temperature for 48 hours, and ethyl acetate was added to the reaction system after the conversion of the substrate Extract (3mL*4), combine the organic phases and dry over anhydrous sodium sulfate, filter and concentrate in vacuo to obtain the crude product. The obtained crude product was purified and separated by column chromatography to obtain a white solid product with an isolated yield of 66%.
[0041] The synthetic route of embodiment 2 is as follows:
[0042]
[0043] The proton nuclear magnetic resonance spectrum of gained target product is as follows:
[0044] 1 H NMR (400MHz, CDCl 3):10.99(s,1H),7.42-7.40(m,2H),7.07–7.01(m,3H),6.65(d,J=5.6Hz,1H),3.44(d,J=10.8Hz,1H) ,3.32(d,J=10.8Hz,1H),1.77(s,3H)....
Embodiment 3
[0046] Add 2,3-dihydroxybenzoic acid (0.3mmol, 46.2mg), 4-methyl-α-methylstyrene (0.15mmol, 21.9uL), MnxEFG (0.01mol %), hydrogen peroxide (0.75mmol, 75uL), deionized water (1mL), acetonitrile (1mL), hydrochloric acid (0.3mmol, 13uL) were magnetically stirred at room temperature for 48 hours, and ethyl acetate was added to the reaction system after the conversion of the substrate Ester extraction (3mL*4), the organic phases were combined and dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to obtain the crude product. The obtained crude product was purified and separated by column chromatography to obtain a white solid product with an isolated yield of 81%.
[0047] The synthetic route of embodiment 3 is as follows:
[0048]
[0049] The proton nuclear magnetic resonance spectrum of gained target product is as follows:
[0050] 1 H NMR (400MHz, CDCl 3 ):11.04(s,1H),7.30(d,J=8.0Hz,2H),7.13(d,J=8.0Hz,2H),7.04(d,J=8.0Hz,1H),6.64(d,J =8.0Hz,1H),5.54(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com