Synthetic method of cyclic dipeptide containing aspartic acid and glutamic acid
A technology of aspartic acid and synthesis method, applied in the field of cyclic dipeptide synthesis, can solve problems such as low safety, air sensitivity, and increased synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
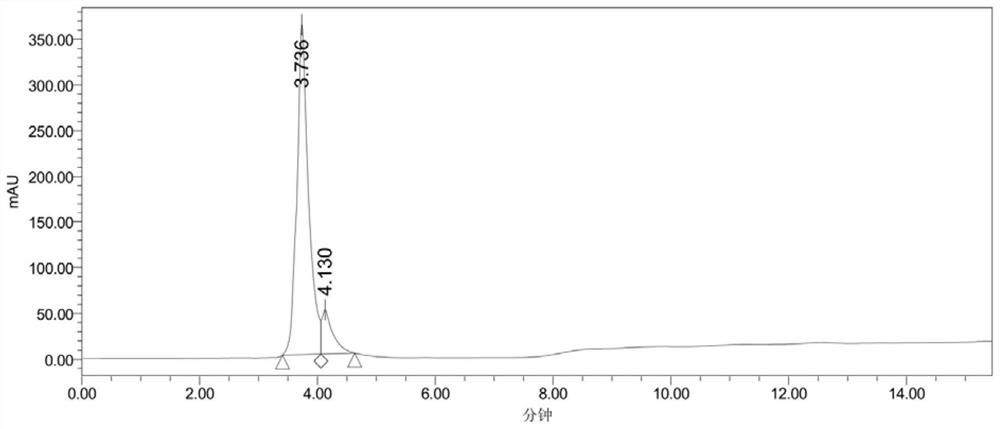
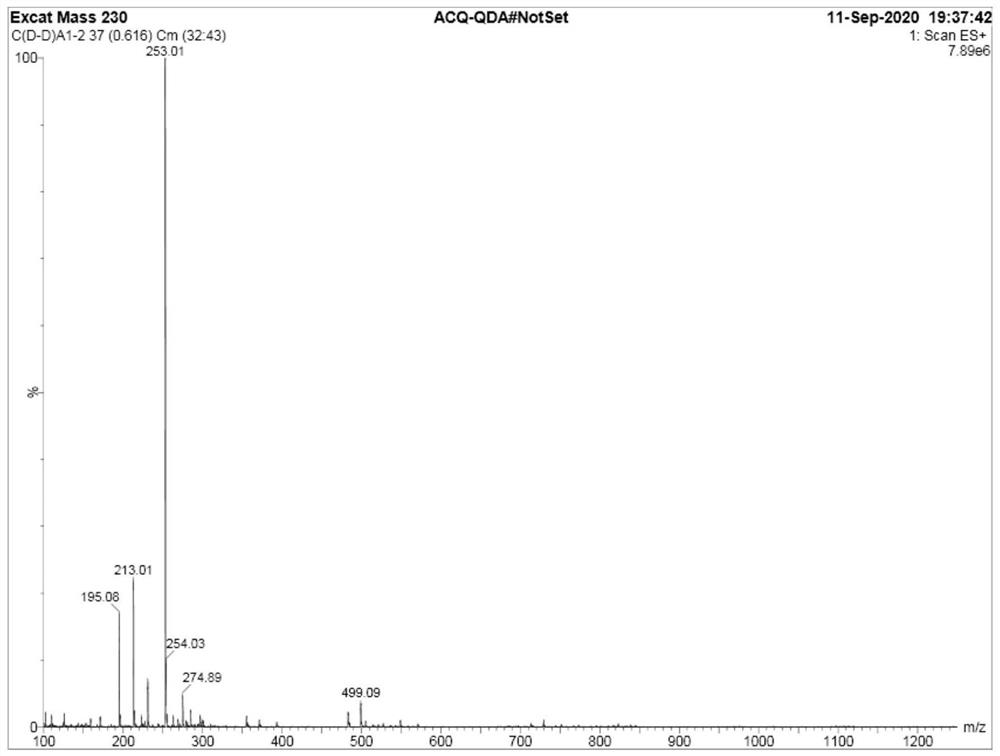
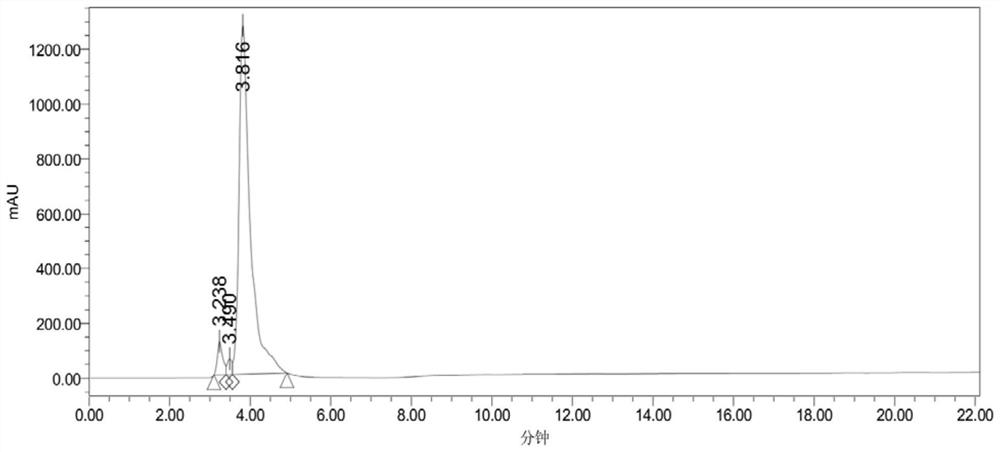
Image
Examples
Embodiment 1
[0044] Synthesis of aspartate-aspartate cyclic dipeptide
[0045] 1. Weigh 40g (97.3mmol) Fmoc-Asp(OtBu)-OH, 21g (107mmol) 9-fluorenemethanol and dissolve in 500mL dry dichloromethane, and add 1.19g (9.73mmol) 4- Dimethylaminopyridine, lower the temperature to 0°C, add dropwise 50mL of dichloromethane solution containing 24.1g (116.8mmol) N,N′-dicyclohexylcarbodiimide, keep the reaction at 0°C for 30 minutes after the dropwise addition, filter The precipitate was removed, the temperature was naturally raised to room temperature, and the reaction was carried out for 7 hours, and the reaction was complete as monitored by TLC. The reaction liquid was concentrated under reduced pressure, and the concentrate was dissolved in a little dichloromethane, and subjected to column chromatography of petroleum ether-ethyl acetate system (volume ratio PE:EA=5:1) to obtain 48 g of white solid Fmoc-Asp(OtBu)-OFm.
[0046] Add 400mL trifluoroacetic acid and 20mL water to 48g (81.4mmol) Fmoc-As...
Embodiment 2
[0054] Synthesis of glutamine-aspartic acid cyclic dipeptide
[0055] Steps 1, 2, and 3 of this embodiment are the same as those of Embodiment 1.
[0056] In step 4 of this example, the H-Asp(2-CTC Resin)-OFm obtained in step 3 was added to 60 mL of N,N-dimethylformamide, and 19 g (32 mmol) of Fmoc-Asn(Trt)- OH, 6.5g (48mmol) 1-hydroxybenzotriazole, 7.4mL (48mmol) N,N'-diisopropylcarbodiimide, and other steps are the same as step 4 of Example 1 to obtain a linear fully protected di Peptide Fmoc-Asn(Trt)-Asp(2-CTC Resin)-OFm.
[0057] Steps 5 and 6 of this embodiment are the same as steps 5 and 6 of Embodiment 1.
[0058]In Step 7 of this example, 92 mL of trifluoroacetic acid, 5 mL of triisopropylsilane, and 3 mL of water were added to Cyclo (Asn(Trt)-Asp(2-CTC Resin)) obtained in Step 6, stirred at room temperature for 2 hours, and then Concentrate under pressure until no fraction overflows, add 30mL of pure water, filter and prepare and purify by hydrophilic chromatograph...
Embodiment 3
[0060] Synthesis of Arginine-Aspartic Acid Cyclic Dipeptide
[0061] Steps 1, 2, and 3 of this embodiment are the same as those of Embodiment 1.
[0062] In step 4 of this example, the H-Asp(2-CTC Resin)-OFm obtained in step 3 was added to 60mL of N,N-dimethylformamide, and 20.8g (32mmol) of Fmoc-Arg(Pbf) was added -OH, 6.5g (48mmol) 1-hydroxybenzotriazole, 7.4mL (48mmol) N,N'-diisopropylcarbodiimide, other steps are the same as step 4 of Example 1, and the linear chain is fully protected Dipeptide Fmoc-Arg(Pbf)-Asp(2-CTC Resin)-OFm.
[0063] Steps 5 and 6 of this embodiment are the same as steps 5 and 6 of Embodiment 1.
[0064] In Step 7 of this example, add 88 mL of trifluoroacetic acid, 5 mL of triisopropylsilane, 4 mL of sulfide anisole, and 3 mL of water to Cyclo (Arg(Pbf)-Asp(2-CTC Resin)) obtained in Step 6, and Stir the reaction for 2 hours, concentrate under reduced pressure until no fraction overflows, add 50mL of pure water, filter and prepare and purify by reve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com