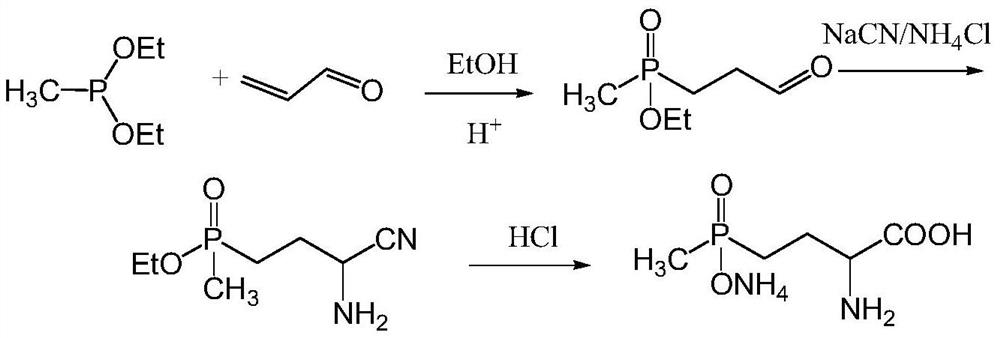
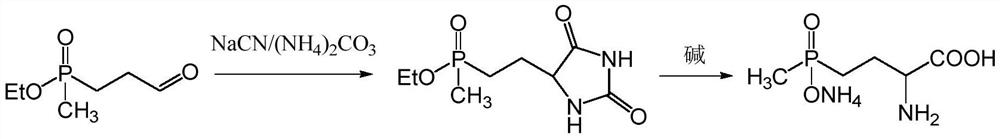
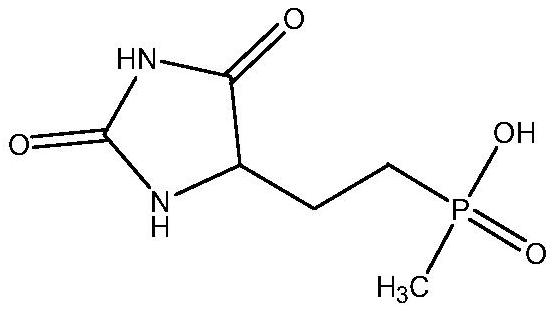
Preparation method of glufosinate-ammonium
A technology of glufosinate-ammonium and hydantoin, applied in the field of pesticides, can solve the problems of high energy consumption and complicated post-treatment of inorganic waste salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The present embodiment provides a kind of preparation method of glufosinate-ammonium, comprising the steps:
[0044] Add 210g (1mol) of 5-[2-(hydroxy(methyl)phosphono)ethyl]hydantoin and 210g of water into a 1L autoclave, then fill it with carbon dioxide to a pressure of 1MPa, and raise the temperature to 120°C (system pressure is 1.4MPa ) to react for 15h. After the completion of the reaction, release carbon dioxide and water vapor (compress and recover while releasing), then concentrate under reduced pressure to 215g, then add 215g methanol, heat and reflux for 2h, crystals are precipitated, filter after cooling, and dry to obtain glufosinate-ammonium 175g, after HPLC Its content is detected to be 96.5%.
[0045] 1 H-NMR (400MHz, D 2 O) δ: 3.64 (t, 1H), 1.90-1.94 (m, 2H), 1.40-1.52 (m, 2H), 1.09 (d, 3H).
[0046] 13 C-NMR (100MHz, D 2 O) δ: 14.46 (d, j=93), 24.16 (d, j=2), 26.51 (d, j=91), 55.05 (d, j=15), 174.13.
Embodiment 2
[0048] The present embodiment provides a kind of preparation method of glufosinate-ammonium, comprising the steps:
[0049] Add 105g (0.5mol) of 5-[2-(hydroxy(methyl)phosphono)ethyl]hydantoin and 1050g water into a 2L autoclave, then fill it with carbon dioxide to a pressure of 4MPa, and raise the temperature to 180°C (the system pressure is 5.5MPa) for 4 hours. After the reaction was completed, carbon dioxide and water vapor were released (compressed and recovered while releasing), then concentrated under reduced pressure to 179g, then added 895g of methanol, and heated to reflux for 2h to have crystallization, filtered after cooling, and dried to obtain glufosinate-ammonium 93.1g. Its content was detected by HPLC to be 98.9%.
Embodiment 3
[0051]The present embodiment provides a kind of preparation method of glufosinate-ammonium, comprising the steps:
[0052] Add 210g (1mol) of 5-[2-(hydroxy(methyl)phosphono)ethyl]hydantoin and 420g of water into a 1L autoclave, then fill it with carbon dioxide to a pressure of 2MPa, and raise the temperature to 140°C (system pressure is 2.8MPa ) to react for 10h. After the reaction was completed, carbon dioxide and water vapor were released (compressed and recovered while releasing), then concentrated under reduced pressure to 263g, then added 526g of methanol, heated to reflux for 2h, crystals were separated out, filtered after cooling, and dried to obtain 192g of glufosinate-ammonium. Its content was detected by HPLC to be 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com