Mouthwash as well as preparation method and application thereof
A mouthwash and solution technology, which is applied in blood diseases, oral care, pharmaceutical formulations, etc., can solve problems such as failure-prone function and singleness, and achieve the effect of enhancing sterilization effect, improving taste, and mild taste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
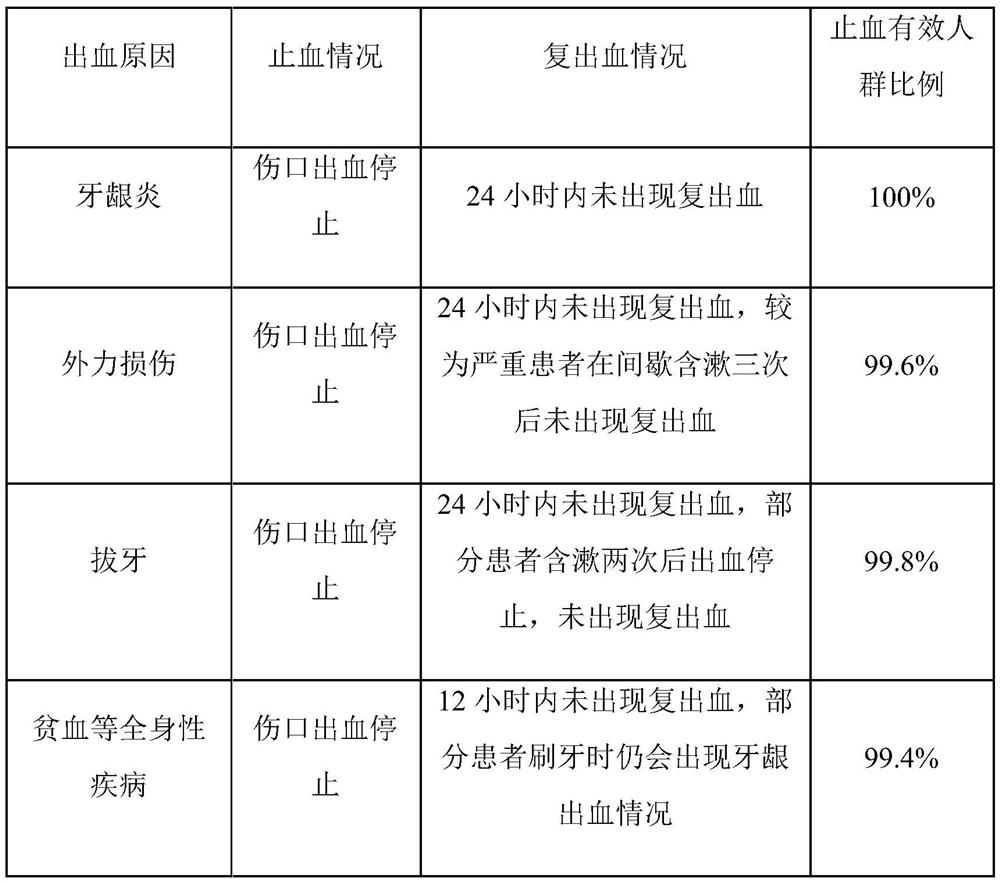
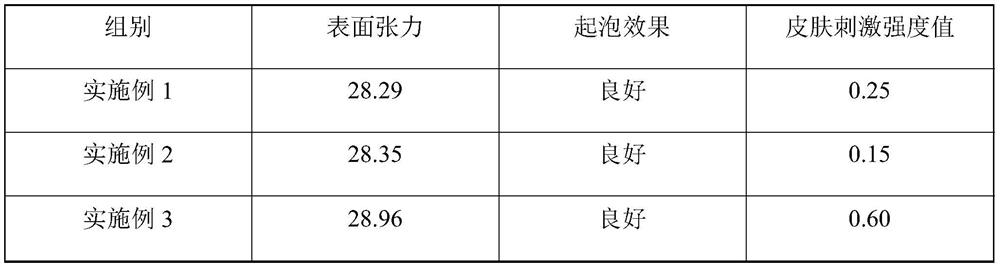
[0038]The present embodiment provides a mouthwash, and every 1 mL of the mouthwash contains the following raw material components: 25 mg of chlorine dioxide solution, 0.1 mg of N-succinyl chitosan, 0.05 mg of sodium ammonium phytate, peppermint Amide 0.4mg, Flavor 0.25mg, Litsea cubeba essential oil 0.2mg, Sodium perfluorononenyloxybenzenesulfonate 7.5mg, Chitosan 0.04mg, Magnesium silicate 0.05mg, Polyaspartic acid 10mg and lemon Zinc acid 15mg, the solvent is deionized water.
[0039] Wherein, the flavoring agent is steviol glycoside and aspartame with a mass ratio of 1:1,
[0040] The mass percent of chlorine dioxide in the chlorine dioxide solution is 1.6%, and its preparation method is: reacting sodium chlorite and a sulfuric acid solution with a volume percent concentration of 10% to generate chlorine dioxide gas and pass through saturated sulfite After the sodium chlorate solution removes impurities, it is obtained by absorbing an aqueous solution containing 1.5g / mL po...
Embodiment 2
[0044] The present embodiment provides a mouthwash, and every 1 mL of the mouthwash contains the following raw material components: 20 mg of chlorine dioxide solution, 0.12 mg of N-succinyl chitosan, 0.07 mg of sodium ammonium phytate, peppermint Amide 6.0mg, Flavor 0.2mg, Litsea cubeba essential oil 0.05mg, Sodium perfluorononenyloxybenzenesulfonate 15mg, Chitosan 0.02mg, Magnesium silicate 0.06mg, Polyaspartic acid 5mg and Citric acid Zinc 60mg, the solvent is deionized water.
[0045] Wherein, the flavoring agent is steviol glycoside and aspartame with a mass ratio of 1.2:1,
[0046] The mass percentage of chlorine dioxide in the chlorine dioxide solution is 1.8%. After the sodium chlorate solution removes impurities, it is obtained by absorbing an aqueous solution containing 1.2 g / mL potassium carbonate and 50% (v / v) hydrogen peroxide.
[0047] The preparation method of above-mentioned mouthwash solution comprises the steps:
[0048] Weigh each component according to th...
Embodiment 3
[0050] The present embodiment provides a kind of mouthwash, and every 1mL described mouthwash contains the raw material components of following quality: chlorine dioxide solution 40mg, N-succinyl chitosan 0.08mg, sodium ammonium phytate 0.04mg, mint Amide 0.2mg, Flavoring Agent 6.0mg, Litsea Cubeba Essential Oil 0.6mg, Sodium Perfluorononenyloxybenzenesulfonate 5mg, Chitosan 0.05mg, Magnesium Silicate 0.03mg, Polyaspartic Acid 25mg and Citric Acid Zinc 10mg, the solvent is deionized water.
[0051] Wherein, the flavoring agent is steviol glycoside and aspartame with a mass ratio of 0.8:1,
[0052] The mass percentage of chlorine dioxide in the chlorine dioxide solution is 1.5%, and its preparation method is: reacting sodium chlorite and a sulfuric acid solution with a volume percentage concentration of 10% to generate chlorine dioxide gas and pass through saturated sulfite After the sodium chlorate solution removes impurities, it is obtained by absorbing an aqueous solution c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com