South America white pair DNA binding antibacterial peptide VPDB40 and application thereof
A technology of antimicrobial peptides and white prawns, applied in the fields of application, antibacterial drugs, peptides, etc., can solve the problems that there is no nucleic acid sequence, recombinant protein research and application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
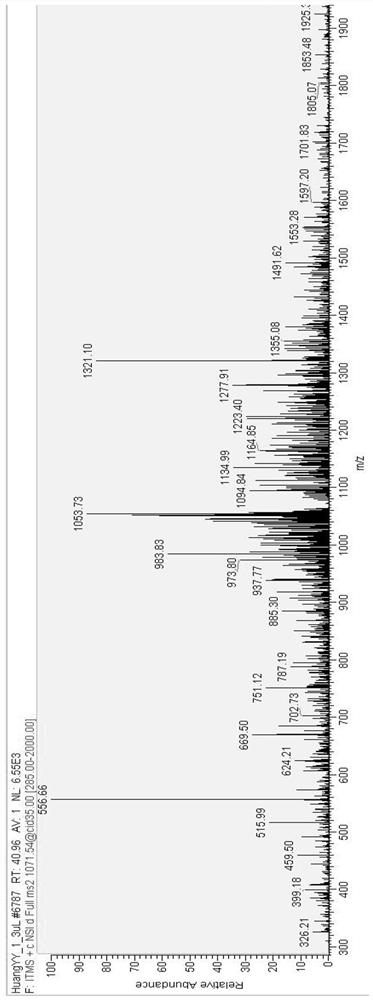
[0041] Example 1 Mass spectrometric analysis of Penaeus vannamei DNA-binding antimicrobial peptides
[0042] Firstly, the samples of Penaeus vannamei were prepared, and 400 healthy Penaeus vannamei were divided into high-intensity ultrasonic group, medium-intensity ultrasonic group, low-intensity ultrasonic group and control group, 100 each. Each shrimp was injected with 0.1mL Vibrio parahaemolyticus (1.0×10 5-6 cfu / mL) or an equivalent amount of sodium chloride. Live shrimp were collected 24 hours after infection and minced immediately with PBS buffer. After centrifuging 100 g for 15 min, the supernatant was taken and passed through a 3000 Da filter membrane. Store at -20°C for further analysis.
[0043] This was then performed using a chromatographic Nano Aquity UPLC system (Waters Corp.) instrument. The injection volume was 5.0 μl, and the mobile phases were 0.1% aqueous methanol and acetonitrile, respectively. The elution gradient is shown in Table 1.
[0044] Table ...
Embodiment 2
[0062] Embodiment 2 Minimum Inhibitory Concentration (MIC) Determination
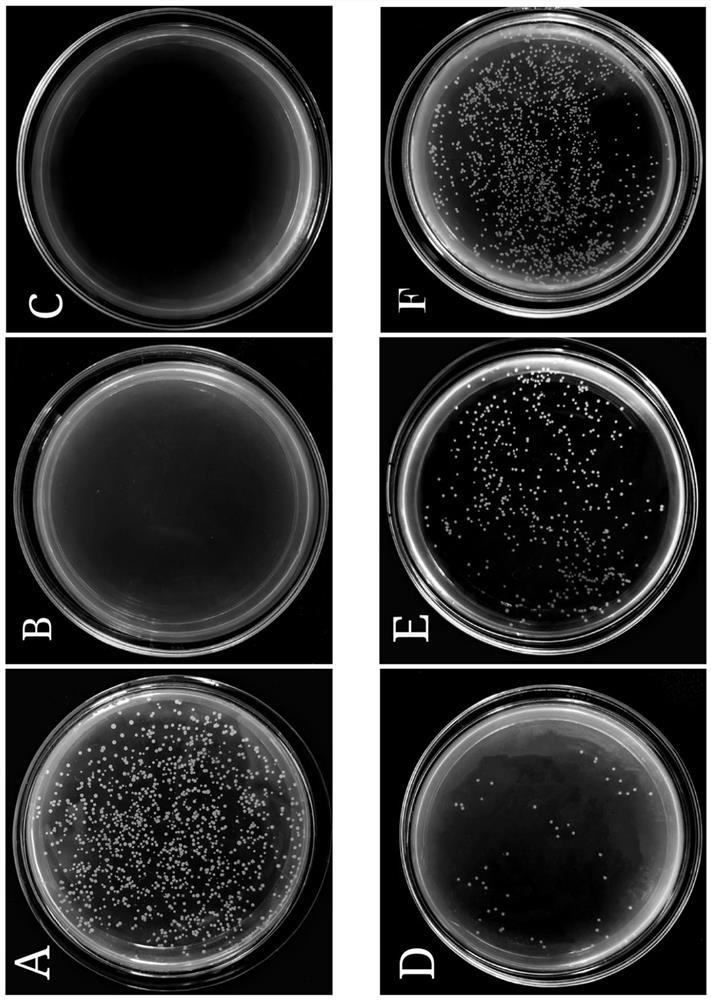
[0063] Cultivate Vibrio parahaemolyticus at 37°C for 12h to logarithmic growth phase, dilute to 10 in 0.01M pH 7.2 phosphate buffer 6-7 CFU / mL. Dissolve the peptide in phosphate buffer, and mix it with bacteria in an equal volume at 37°C for 2 hours. The minimum inhibitory concentration (MIC) refers to the lowest concentration of antimicrobial peptide at which no bacterial growth can be seen from the microtiter plate after incubation at 37°C. like figure 2 As shown, the minimum inhibitory concentration (MIC) of VPDB40 to Vibrio parahaemolyticus was 1.95 μg / mL.
Embodiment 3
[0064] Embodiment 3 time-killing curve (Time kill) is measured
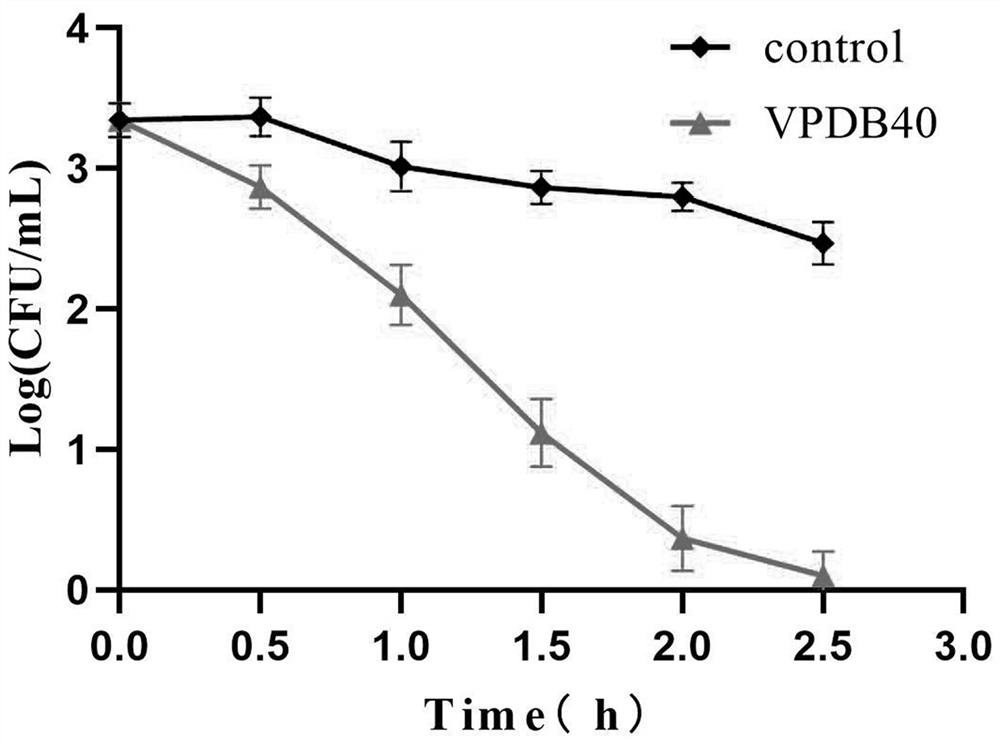
[0065] The time-kill curve of peptide VPDB40 was determined using the plate count method. Cultivate Vibrio parahaemolyticus at 37°C for 12h to logarithmic growth phase, dilute to 10 in 0.01M pH 7.2 phosphate buffer 6-7 CFU / mL. The peptide was dissolved in phosphate buffer and diluted to 3.91 μg / mL, mixed with bacteria in equal volume, and incubated at 37°C. At different time points (ie, 0.5, 1, 1.5, 2, 2.5 and 3 o'clock), 0.2 mL of bacterial suspension was obtained, and colonies were counted after 24 hours of culture on a nutrient broth plate at 37°C. like image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com