Clean preparation method of high-purity methionine hydroxyl analogue calcium salt
A technology of high-purity methionine hydroxyl and methionine hydroxyl, which is applied in the field of clean preparation of high-purity methionine hydroxyl analog calcium salt, can solve the problems of ammonium sulfate unpleasant smell, loss of organic solvent, large investment in equipment, etc., to avoid by-product inorganic The effect of ammonium salt generation, reduction of solid waste generation, and high monomer content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
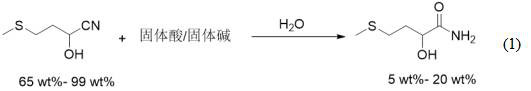
[0026] The clean preparation method of a kind of high-purity methionine hydroxyl analogue calcium salt of the present invention comprises the following steps:
[0027]1) Use 2-hydroxy-4-methylthiobutyronitrile as raw material, add water, and carry out hydration reaction under the catalysis of solid acid or solid base to obtain 2-hydroxy-4-methylthiobutyroamide salt solution;
[0028] 2) adding strong acid or strong base to the 2-hydroxy-4-methylthiobutyramide saline solution for hydrolysis to obtain 2-hydroxy-4-methylthiobutyric acid;
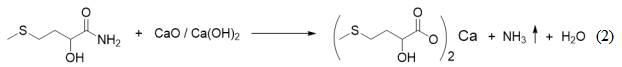
[0029] 3) Add CaO or Ca(OH) to the 2-hydroxy-4-methylthiobutyric acid 2 , through a salt-forming reaction, the aqueous solution of the mixture and ammonia are obtained, and the ammonia is absorbed by water to obtain ammonia water.
[0030] 4) The aqueous solution of the mixture is subjected to chromatographic desalination treatment to obtain an aqueous solution of methionine hydroxyl analogs and an aqueous solution of inorganic ammonium salt; ...
Embodiment 1
[0048] 30g ZrOCl 2 · 8H 2 O, add 45 mL H 2 SiO 3 dissolve, add NH 3 ·H 2 O to adjust pH=8, add 10g of powdered activated carbon, fully stir at 100°C for 1 hour, age, and wash with suction until no Cl - , Dry at 80°C for 10h. h 2 SO 4 Soak in water for 3 hours, filter, wash, and dry, and then roast at 300°C for 2 hours to obtain activated carbon-loaded SO 4 2- / SiO 2 -ZrO 2 New solid superacid catalyst.
Embodiment 2
[0050] 30g ZrOCl 2 · 8H 2 O, add 45 mL H 2 SiO 3 dissolve, add NH 3 .H 2 O to adjust pH=8, add 15g of powdered activated carbon, fully stir at 100°C for 1 hour, age, filter and wash until no Cl - , Dry at 80°C for 10h. h 2 SO 4 Soak in water for 3h, filter and wash, dry and roast at 300°C for 2h to obtain SO 4 2- / SiO 2 -ZrO 2 Solid superacid catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com