Preparation method of progesterone
A technology of progesterone and ketal, applied in the direction of steroids, organic chemistry, etc., can solve the problems of operator poisoning, unsuitable for industrialization, and low yield, and achieve the effects of high safety factor, environmental friendliness, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
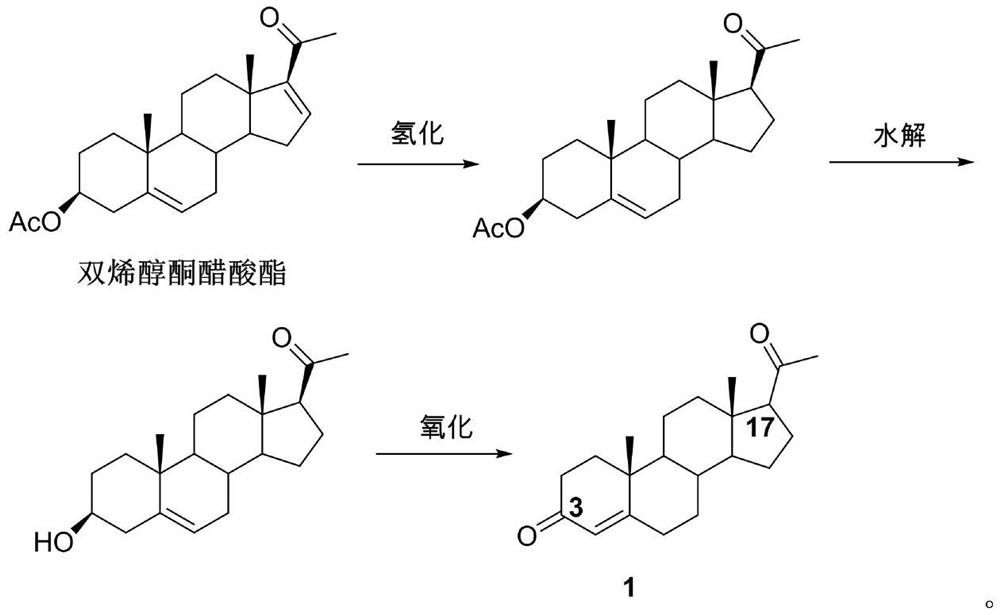
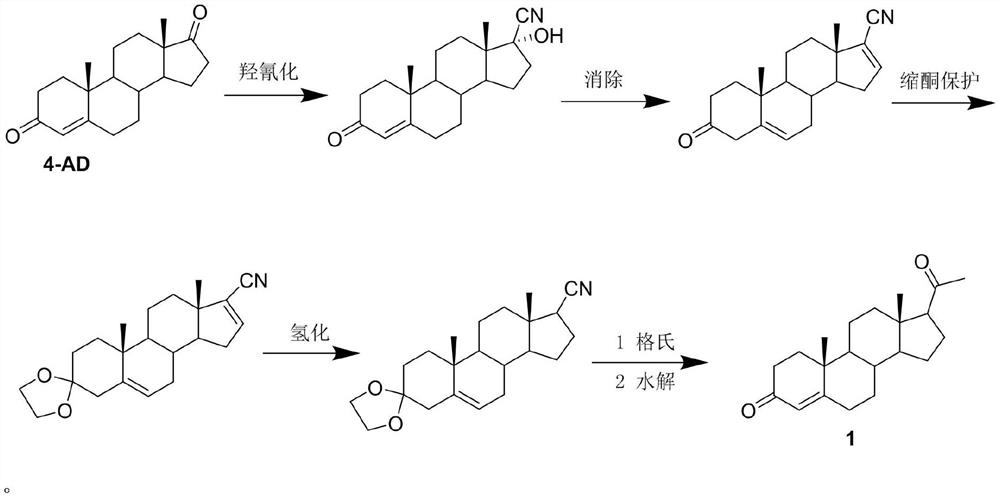
Image
Examples
Embodiment 1
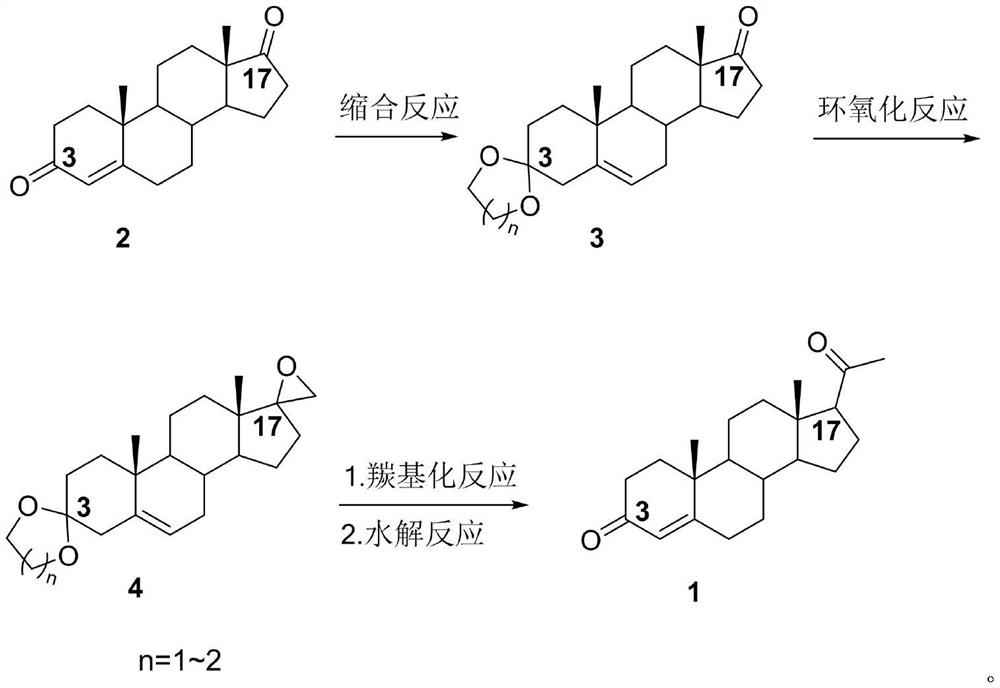
[0055] The preparation of embodiment 1 formula 3 compound
[0056]
Embodiment 1-1
[0058] Under the protection of nitrogen, add 50mL dichloromethane, 10g4-AD (compound 2), 2.6mL ethylene glycol, 11.7mL triethyl orthoformate and 0.01g p-toluenesulfonic acid into the reaction flask, react at 20°C, TLC No compound of formula 2 was detected. After the reaction was completed, triethylamine was added to quench the reaction, and concentrated under reduced pressure to 2-3 times the volume. After cooling to room temperature, the concentrate was diluted into ice water, filtered, and dried to obtain 11.3 g of a compound of formula 3 as an off-white solid. Yield 97.9%, HPLC content 97.0%.
Embodiment 1-2
[0060] Under the protection of nitrogen, add 55mL chloroform, 10g4-AD (compound 2), 2.3mL ethylene glycol, 6.5mL trimethyl orthoformate and 0.01g p-toluenesulfonic acid to the reaction flask, react at 25°C, TLC No compound of formula 2 was detected. After the reaction is complete, add pyridine to quench the reaction, concentrate under reduced pressure to 2-3 times the volume, and after cooling down to room temperature, dilute the concentrate into ice water, filter, and dry to obtain 11.2 g of off-white solid formula 3, with a yield of 97.1 %, HPLC content 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com