A kind of synthetic method of cytochalasin compound flavipesine A
A technology for the synthesis of cytochalasin and its synthesis method, which is applied in the field of synthesis of the cytochalasin compound flavipesineA, which can solve the problems of high cost, long cycle, and expensive price, and achieve the effects of low cost, short production cycle, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
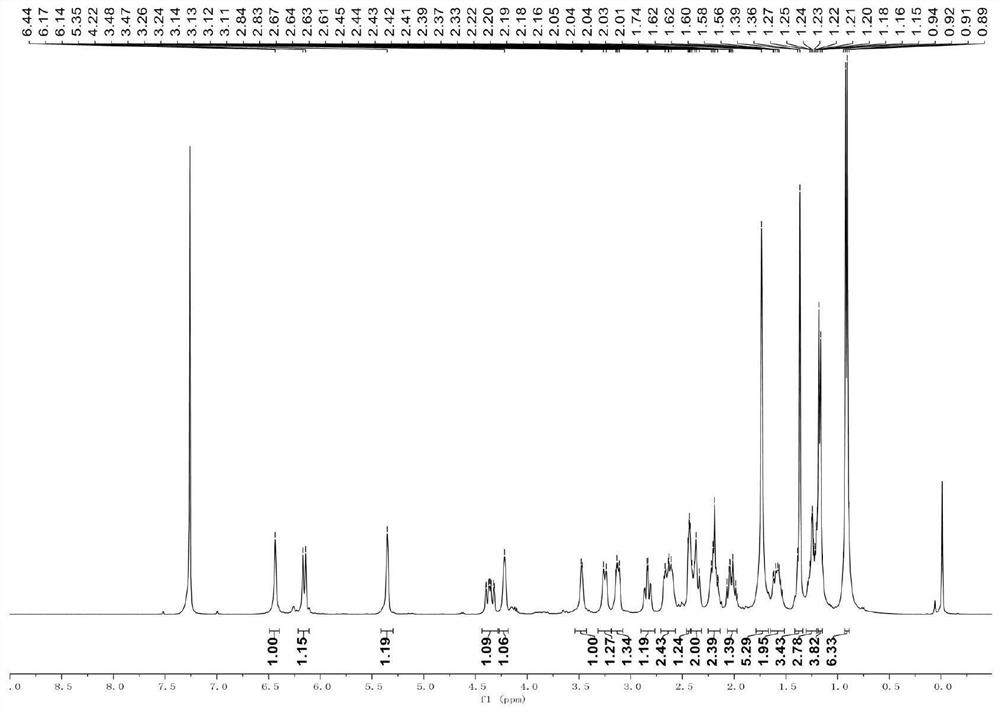
[0032] Embodiment 1 of the present invention provides the synthetic method of compound B used in the present invention, which is as follows:
[0033]
[0034] P-toluenesulfonic acid (2.27 mmol) and 2,2,6,6-tetramethylpiperidine oxide (2.34 mmol) were mixed in 15 mL of dichloromethane solution under ice bath, and continued stirring (400 rpm) for 10 min under ice bath Then, the dichloromethane solution of this mixture was added to a 7 mL dichloromethane solution of compound D (0.38 mmol), and the stirring was continued (400 rpm) for 1 h under an ice bath, and then the reaction was quenched with 10 mL of saturated aqueous sodium bicarbonate solution under an ice bath. (10 min), extracted with ethyl acetate at room temperature, washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered under reduced pressure (500 mbar), and concentrated under reduced pressure (150 mbar), the obtained crude product was used in a silica gel column layer ...
Embodiment 2
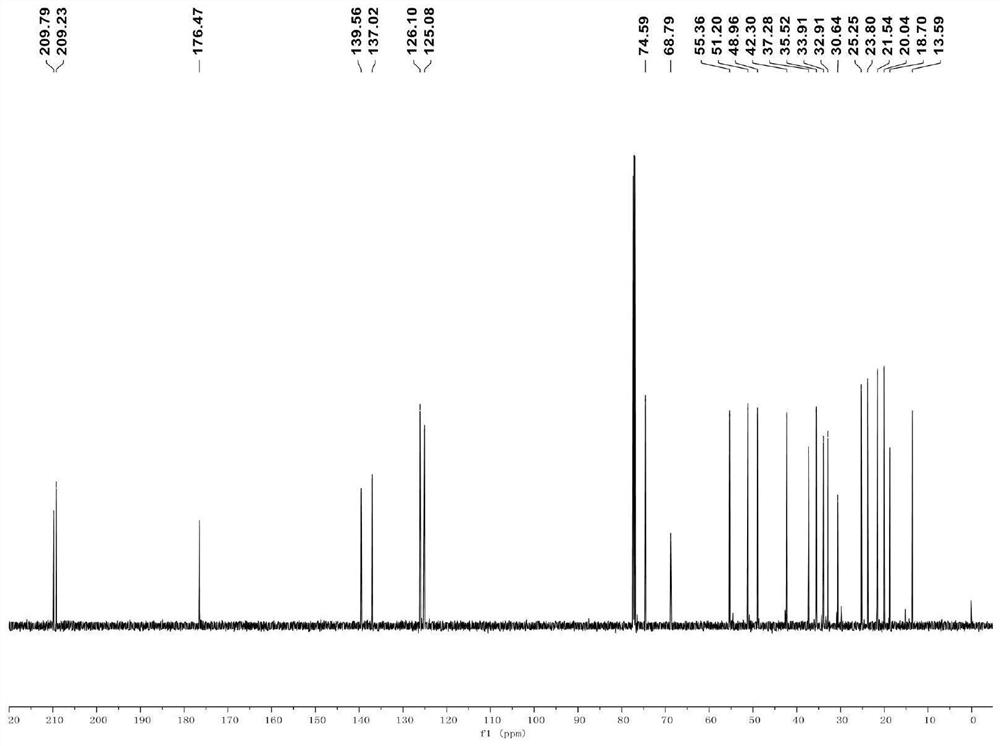
[0040] A method for synthesizing a cytochalasin compound flavipesine A, the specific synthesis route of which is as follows:
[0041]
[0042] Specific steps are as follows:
[0043] (1) Compound B (0.075 mmol) was dissolved in 1.5 mL of anhydrous tetrahydrofuran at room temperature, zinc powder (0.90 mmol) and ammonium acetate (1.05 mmol) were added, and then stirred at 40° C. (450 rpm) for 2 h, and then At room temperature, filtered through celite (500 mbar) and concentrated under reduced pressure (150 mbar) to obtain the crude product, which was purified by silica gel column chromatography (eluent: V ethyl acetate: V petroleum ether=50:50) to obtain intermediate C (26.9 mg, 89% yield);
[0044] (2) At room temperature, dissolve intermediate C (0.05 mmol) in 1 mL of anhydrous tetrahydrofuran, add scandium trifluoromethanesulfonate (0.25 mmol), stir (450 rpm) for 2 h, and then use 1 mL of saturated sodium bicarbonate at room temperature The reaction was quenched with aqu...
Embodiment 3
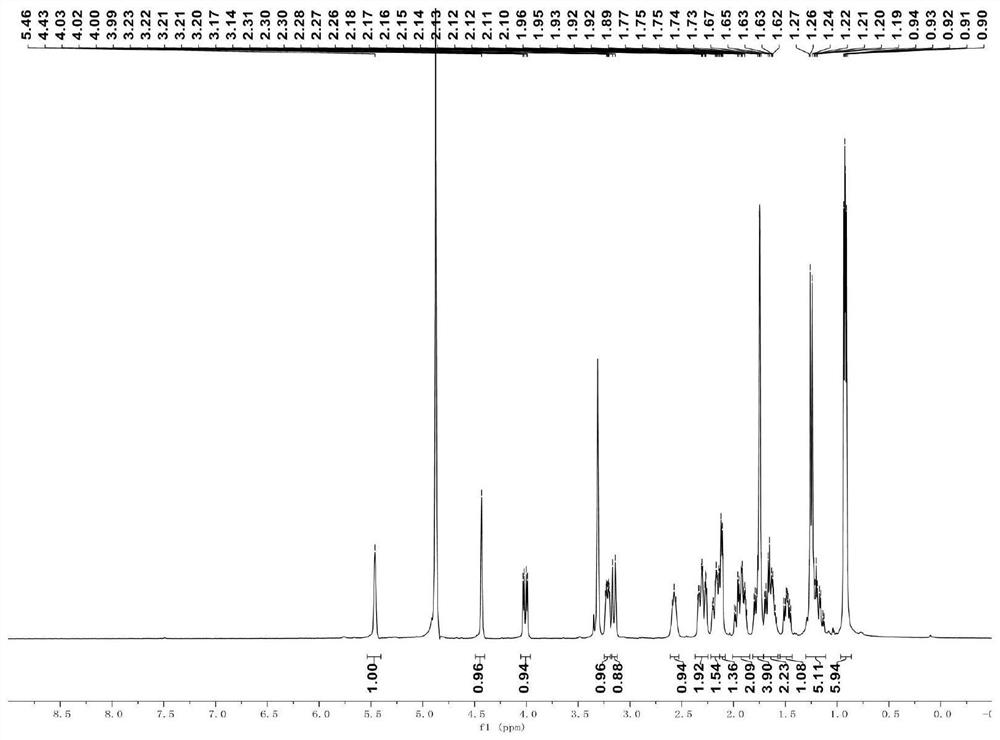
[0046]
[0047] (1) Compound B (0.075 mmol) was dissolved in 0.15 mL of anhydrous tetrahydrofuran at room temperature, zinc powder (0.75 mmol) and ammonium acetate (0.90 mmol) were added, and then stirred at 40° C. (450 rpm) for 2 h, and then Filtered through celite (500 mbar) at room temperature and concentrated under reduced pressure (150 mbar) to obtain the crude product, which was purified by silica gel column chromatography (V ethyl acetate: V petroleum ether = 50:50) to give Intermediate C (22 mg, yield 73%);
[0048] (2) At room temperature, dissolve intermediate C (0.05 mmol) in 0.1 mL of anhydrous tetrahydrofuran, add scandium trifluoromethanesulfonate (0.20 mmol), stir (450 rpm) for 2 h, and then add 1 mL of saturated hydrogen carbonate at room temperature The reaction was quenched with aqueous sodium solution (10 min), extracted with ethyl acetate, washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com