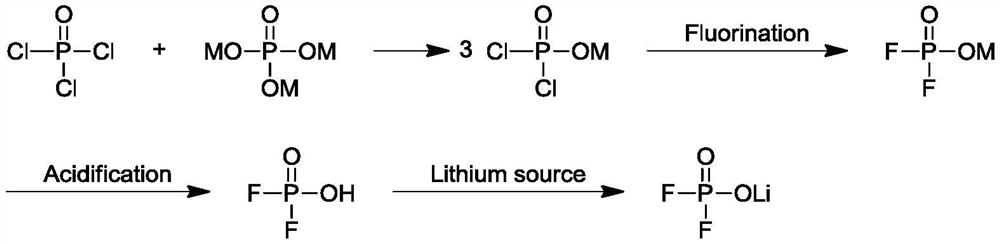
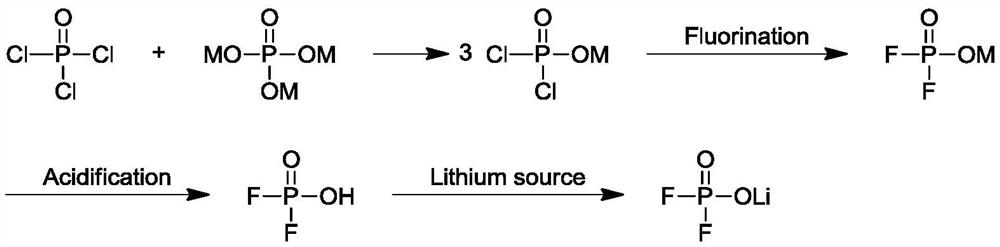
Preparation method of lithium difluorophosphate
A technology of lithium difluorophosphate and difluorophosphoric acid, which is applied in the field of electronic chemicals, can solve problems such as difficult separation and purification, high reaction temperature, and complicated operation, and achieve rapid and thorough reaction, mild reaction conditions, and strong operability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of sodium dichlorophosphate:
[0030] Add 164.0g of anhydrous trisodium phosphate (1.0mol) into a 3.0L four-neck flask, then add 2.0L of anhydrous acetonitrile, and slowly add 322.0g of phosphorus oxychloride (2.1mol) dropwise at room temperature, and there is exotherm during the dropping process . After the addition, the oil bath was heated to reflux temperature and stirred vigorously for 4h. After cooling, the reaction mixture was filtered, and the filtrate was concentrated to obtain 402.3 g of white solid, namely sodium dichlorophosphate, with a yield of 85.4%.
[0031] (2) Preparation of sodium difluorophosphate:
[0032] Add 402.3g of sodium dichlorophosphate (2.56mol) and 2.0L of anhydrous acetonitrile into a 3.0L four-neck flask, add 322.5g of anhydrous NaF (7.68mol) spray-dried in batches at reflux temperature, and stir vigorously at this temperature for 5 hours. After cooling, the reaction mixture was filtered, and the filtrate was concentrat...
Embodiment 2
[0038] (1) Preparation of Potassium Dichlorophosphate:
[0039] Add 212.3g of anhydrous tripotassium phosphate (1.0mol) into a 3.0L four-neck flask, then add 2.0L of anhydrous acetonitrile, and slowly add 322.0g of phosphorus oxychloride (2.1mol) dropwise at room temperature, and there is exotherm during the dropping process . After the addition, the oil bath was heated to reflux temperature and stirred vigorously for 4h. After cooling, the reaction mixture was filtered, and the filtrate was concentrated to obtain 451.5 g of white solid, namely potassium dichlorophosphate, with a yield of 87.0%.
[0040] (2) Preparation of Potassium Difluorophosphate:
[0041] Add 451.5g of potassium dichlorophosphate (2.61mol) and 2.0L of anhydrous acetonitrile into a 3.0L four-neck flask, add 454.9g of spray-dried anhydrous KF (7.83mol) in batches at reflux temperature, and stir vigorously at this temperature for 5h. After cooling, the reaction mixture was filtered, and the filtrate was c...
Embodiment 3
[0047] (1) Preparation of sodium dichlorophosphate:
[0048] Add 164.0g of anhydrous trisodium phosphate (1.0mol) into a 3.0L four-neck flask, then add 2.0L of anhydrous acetonitrile, and slowly add 322.0g of phosphorus oxychloride (2.1mol) dropwise at room temperature, and there is exotherm during the dropping process . After the addition, the oil bath was heated to reflux temperature and stirred vigorously for 2h. After cooling, the reaction mixture was filtered, and the filtrate was concentrated to obtain 353.5 g of white solid, namely sodium dichlorophosphate, with a yield of 75.0%.
[0049] (2) Preparation of sodium difluorophosphate:
[0050] Add 353.5g of sodium dichlorophosphate (2.25mol) and 2.0L of anhydrous acetonitrile into a 3.0L four-neck flask, add 283.4g of anhydrous NaF (6.74mol) spray-dried in batches at reflux temperature, and stir vigorously at this temperature for 3h. After cooling, the reaction mixture was filtered, and the filtrate was concentrated to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com