Compound used as hexanokinase inhibitor and application thereof
A compound and pharmaceutical technology, applied in the field of drug research and development, can solve problems such as inflammation, liver fibrosis, and unmet clinical needs, and achieve the effect of improving activity and pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 2- (1-methyl-3- (2 - ((S) -2-methyl nitric hetericycloone-1-yl) -6- (trifluoromethyl) pyrimidine-4-yl) -3- Nitrogen and bicyclic ring [3.1.0] hexane-6-yl) acetic acid structure is as follows:
[0053]
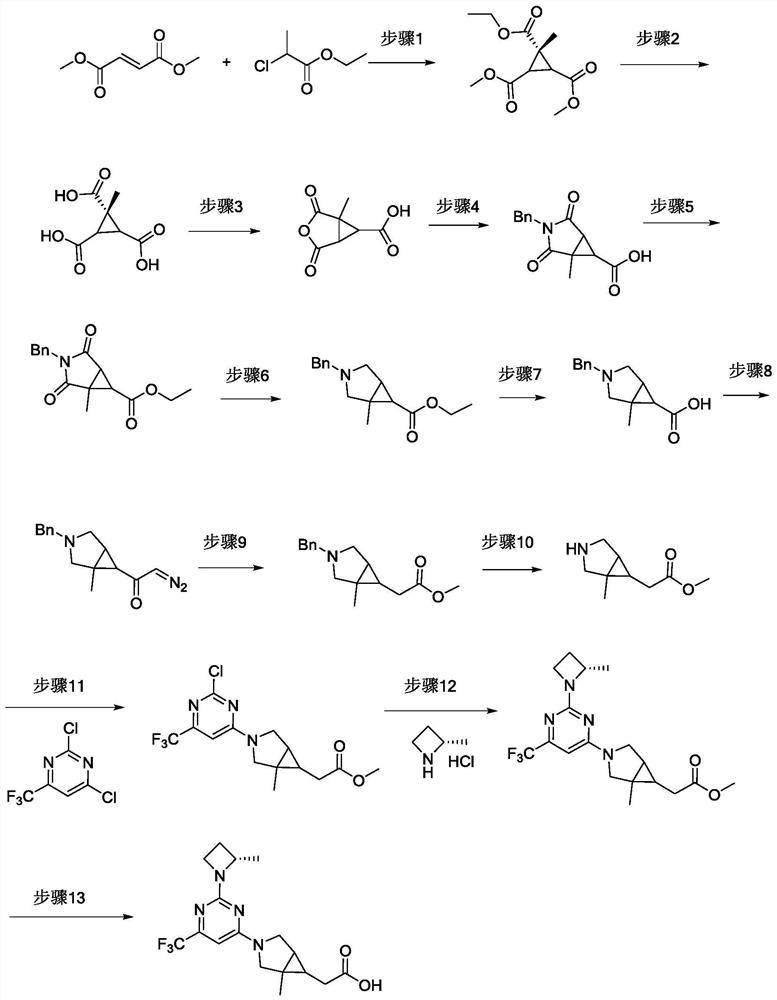
[0054] The synthetic roadmap of the compound of this embodiment is attached figure 1 As shown, the specific preparation method of the compound of the present embodiment includes:
[0055] Step 1: 1-methylcyclopropane-1-methylene-2,3-formate
[0056] Effermate (10.01 g, 69.38 mmol), benzyl triethyl ammonium chloride (0.16 g, 0.69 mmol) was added to NaH (2.16 g, 90.2 mmol) DMF (100 mL) solution. Ethyl 2-chloropropionate (10.42 g, 76.32 mmol) was slowly added dropwise, and the reaction was stirred at 40 ° C overnight;
[0057] The reaction solution was poured into ice water (30 mL), and methyl tert-butyl ether (30 mL × 2) was extracted, combined with organic phase, saturated brine (80 mL) wash, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure...
Embodiment 2
[0093] 2- (1-methyl-3- (2 - ((S) -2-methyl nitric hetericycloone-1-yl) -6- (trifluoromethyl) pyrimidine-4-yl) -3- Nitrogen and mixed bicyclic ring [3.1.0] hexane-6-yl-2, 2, 4, 4-d 4 Acetic acid, structural formula is as follows:
[0094]
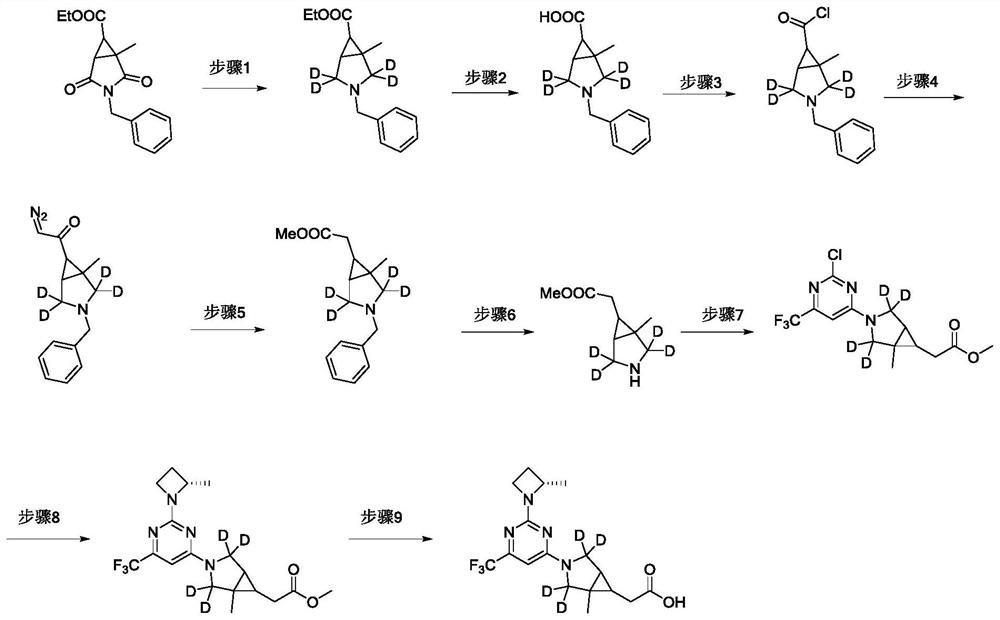
[0095] The synthetic roadmap of the compound of this embodiment is attached figure 2 As shown, the specific preparation method of the compound of the present embodiment includes:
[0096] Step 1: 3-benzyl-1-methyl-3-nitroza bicyclic ring [3.1.0] hexane-6-methylene-2, 2, 4, 4-D 4
[0097] At 0 ° C, the boronide (2.2.10 g, 73.18 mmol, 47%), dripped, slowly dropped into the example, slowly dropped into the example, dripped, slowly dropped into the examples in a solution of sodium borohydride (2.3 g, 54.89 mmol). 1: 3-benzyl-1-methyl-2,4-dioxane-3-nitrogen-1-methyl-2,4-dioxane-3-nitrogen-1-methyl-2,4-dioxy-3-nitrogen-1-methyl-2,4-dioxane-6-formate (5.0 g, 18.03 mmol) tetrahydrofuran (30 mL) solution, drip, and mixed at room temperature overnight....
Embodiment 3
[0120] 2- (1-ethyl-3- (2 - ((S) -2-methyl nitric hetericycloone-1-yl) -6- (trifluoromethyl) pyrimidine-4-yl) -3- Nitrogen-mixed bicyclic ring [3.1.0] hexane-6-yl) acetic acid, structural formula is as follows:
[0121]
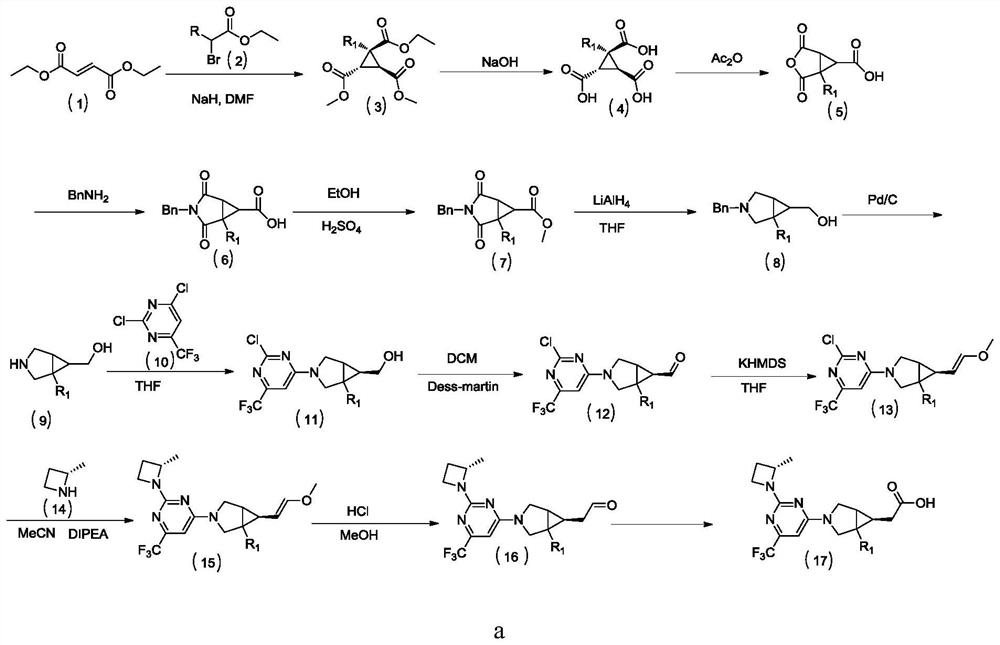
[0122] The specific preparation method of the compound of the present embodiment includes:
[0123] Step 1: 1-ethylcyclopropane-1,2,3-trimethoate
[0124] Epiga group (15.0 g, 104.10 mmol) was added to NaH (6.2 g, 156.50 mmol) DMF (100 mL) solution, and 2-bromobutyrate (20.3 g, 104.10 mmol) was slowly added dropwise. ), 60 ° C agitation reaction overnight. The reaction solution was poured into ice water (300 mL), ethyl acetate (300 ml × 2) extracted, combined with organic phase, saturated brine (100 mL) wash, dried over anhydrous sodium sulfate, filtered, concentrated, and the title compound was pale yellow The oil is 13.0 g of the oil, which is not used directly in the next step without further purification.
[0125] Step 2: 1-ethylcyclopropane-1,2,3-trimethoic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com