Monomer for polymer gel, polymer gel and preparation method
A polymer gel and monomer technology, applied in the direction of sulfate ester preparation, chemical instruments and methods, alkali metal oxides/hydroxides, etc., can solve the problem of difficult to accurately predict solvent molecules and polymer main chain and side chain groups Group interaction etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of embodiment one TPC-OTBS
[0032]
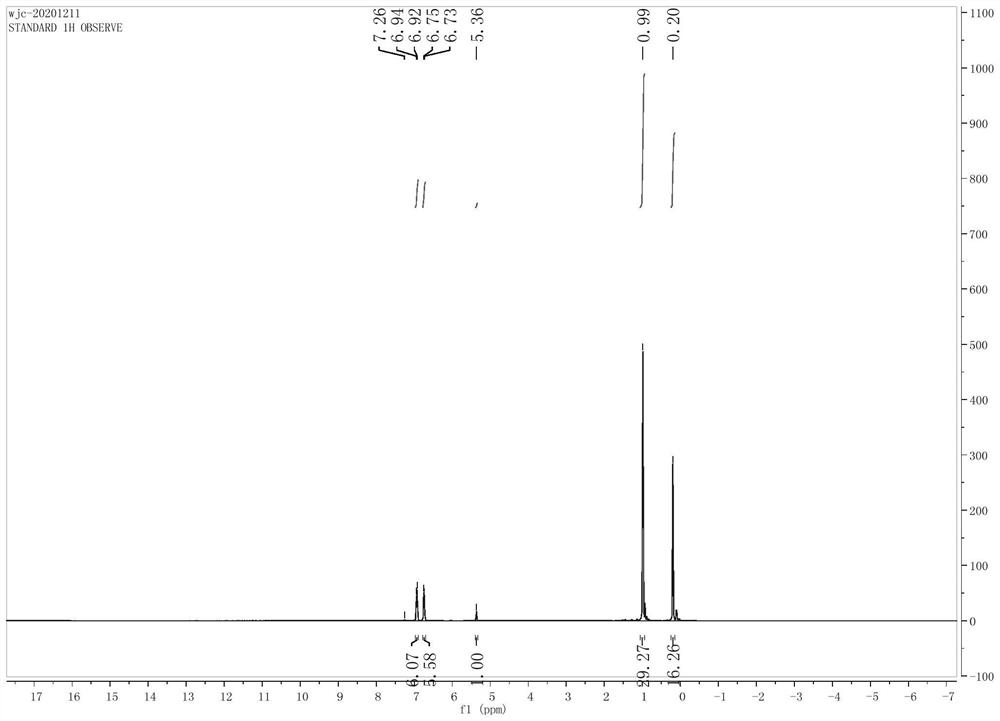
[0033]4,4',4''-trihydroxytriphenylmethane (1.46 g, 5 mmol) and imidazole (1.36 g, 20 mmol) were placed in a 100 mL flask, 20 mL of dichloromethane was added, and stirred at room temperature for 15 minutes, The solid dissolved completely. Dissolve tert-butyldimethylsilyl chloride (TBSCl, 3.02 g, 20 mmol) in 10 mL of dichloromethane, and add it dropwise to the above-mentioned flask through a constant pressure dropping funnel, and keep regular stirring in the flask during the dropwise addition, 30 Minutes dropwise completed. The whole reaction was stirred for 12 hours at room temperature. Use TLC to detect the progress of the reaction. After the conversion of the raw materials, the solids are removed by filtration. After the filtrate is spin-dried, the crude product is purified by column chromatography. The developer is dichloromethane / petroleum ether (v / v=1 / 2), and the pure product It is a pure white solid (2.3 g, ...
Embodiment 2
[0034] Embodiment 2 TPC-OSO 2 Synthesis of F
[0035]
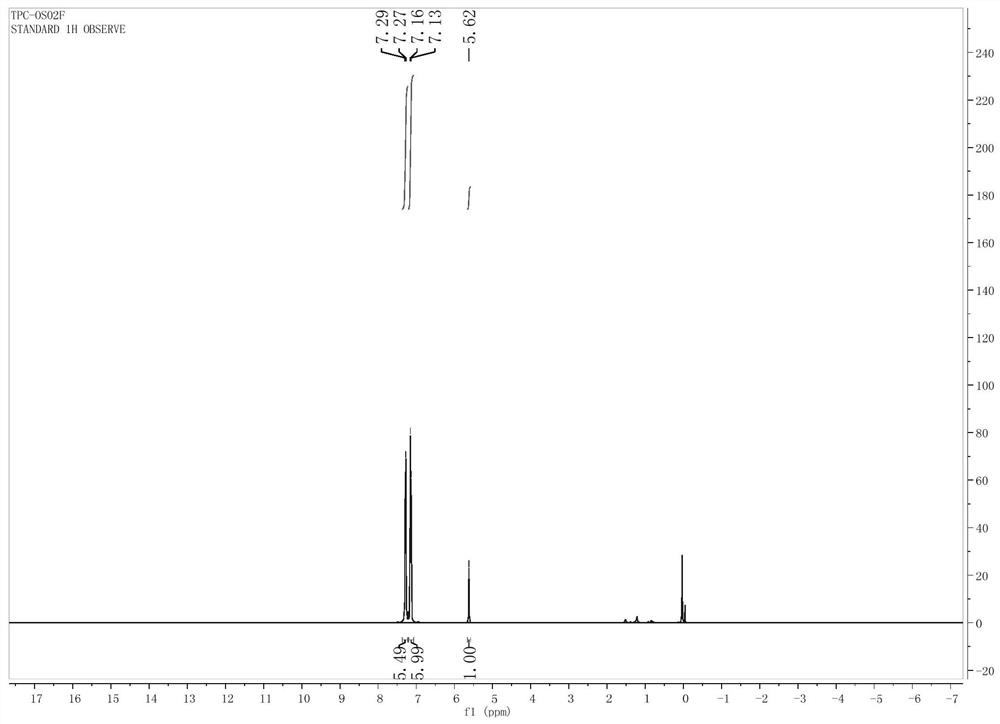
[0036] 4,4',4''-trihydroxytriphenylmethane (1.46 g, 5 mmol) was placed in a 1000 mL flask, 20 mL of dichloromethane was added, and triethylamine (2.1 g, 20 mmol), stirring continuously to dissolve all solids. Seal the flask, pump it to a vacuum with a water pump, and then inject sulfuryl fluoride gas with a 55 L air bag. The whole reaction system was kept sealed and stirred for 12 hours at room temperature. The degree of progress of the reaction was detected by TLC. After conversion of the raw materials, the solid was removed by filtration, and the crude product was purified by column chromatography after the filtrate was spin-dried. The developer was ethyl acetate / petroleum ether (v / v=1 / 4). The pure product is white fine crystals (2.5 g, yield: 93%) TPC-OSO 2 F. The NMR spectrum of the synthesized product is attached figure 2 . 1 H NMR (400 MHz, CDCl 3 ,ppm) δ 7.28 (d, J = 8.6 Hz, 6H), 7.16 (d, J = 8.7 Hz...
Embodiment 3
[0037] Example 3 Preparation of TPC-cPS-gel
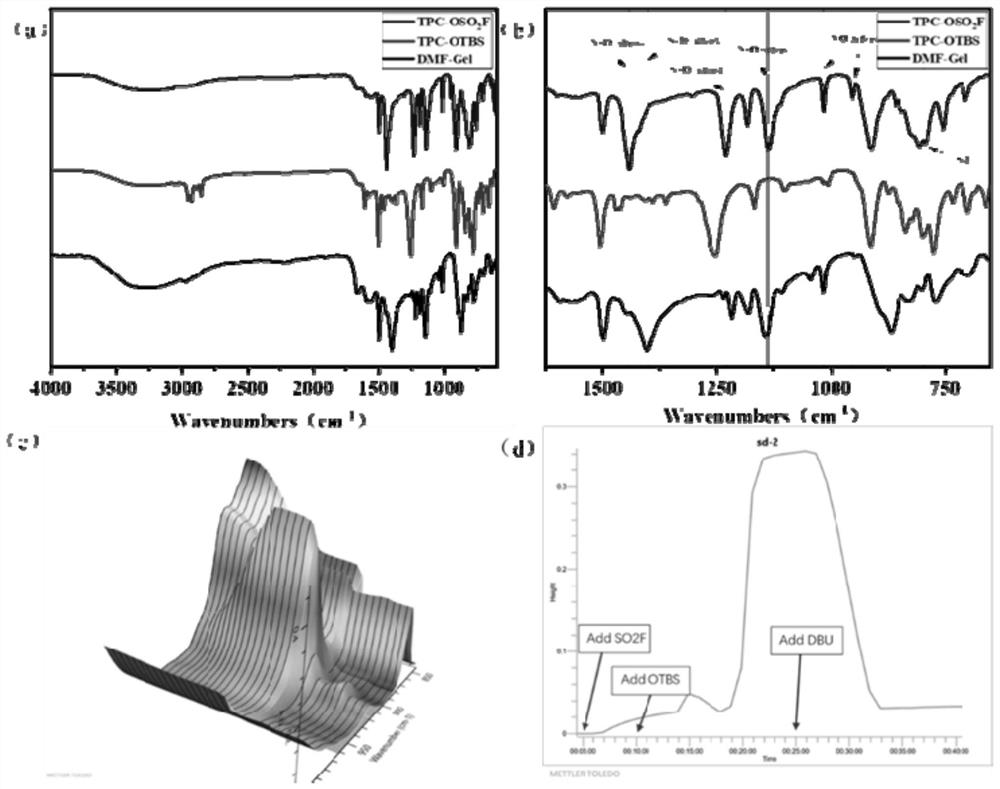
[0038] At room temperature, weigh an equimolar amount of TPC-OSO 2 Add F (200 mg) and TPC-OTBS (235 mg) into a 20 mL sample bottle, use a pipette gun to measure 4 mL of DMF and add it to the sample bottle as a solvent, dissolve all solids by conventional ultrasound, and then add 50 ug of DBU, sonicate again to dissolve and disperse evenly, let the sample bottle stand for 24 hours, pour off the upper layer of liquid, take the lower layer of gel, it is TPC-cPS-gel, and the formed colorless gel does not settle by centrifugation. image 3 for TPC-OSO 2 F, FT-IR of TPC-OTBS, TPC-cPS-Gel, image 3 a. image 3 b is the in situ infrared image of TPC-cPS-Gel, image 3 c. image 3 d; at 1437cm -1 、1219cm -1 Two characteristic stretches are observed at , which correspond to the S=O symmetric and asymmetric stretching vibration peaks, 1012cm -1 、940cm -1 The absorption peak is the stretching peak of S-O, 816cm -1 The peak at is attri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com