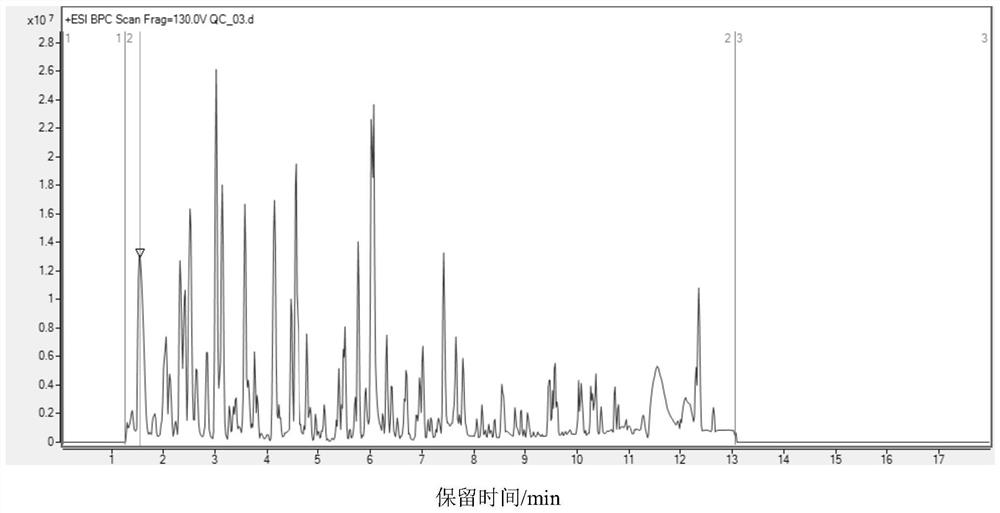
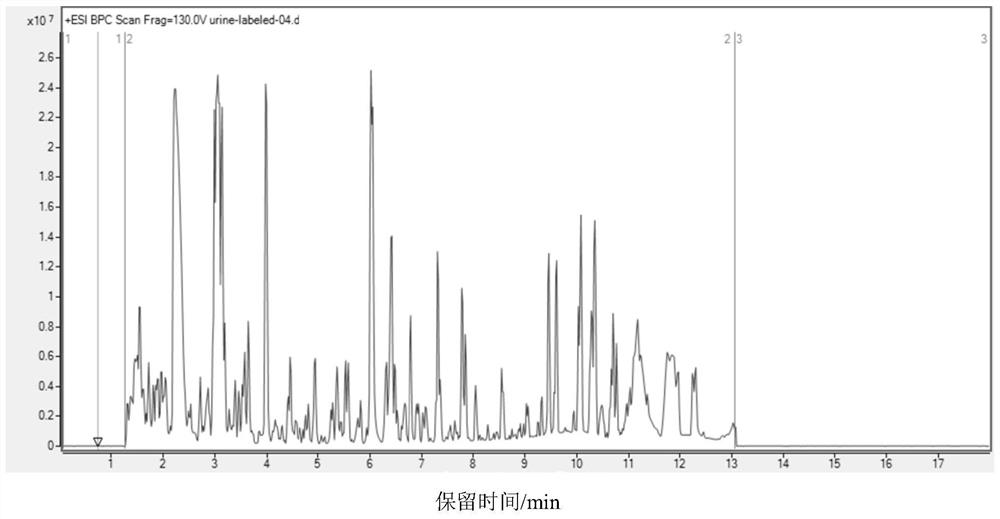
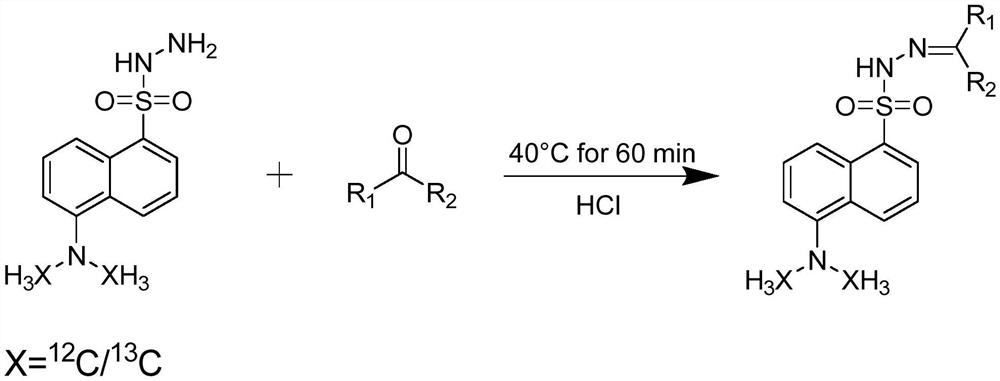
Carbonyl metabolite derivatization method and non-targeted metabonomics efficient analysis method
A derivatization and metabolite technology, applied in the field of metabolomics analysis, can solve the problem that the sensitivity needs to be improved, and achieve the effect of increasing detection sensitivity, eliminating noise interference and reducing instrument drift.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Take a tissue sample, weigh the tissue, and place it in a centrifuge tube. If the sample is too large, cut the original sample so that the weight of the sample is around 150-200mg. Add pre-cooled methanol / water solution to the centrifuge tube, according to the ratio of 500 μL methanol / water solution: 100 mg tissue, the ratio of methanol / water solution is 4:1, v / v. Homogenize the tissue sample for 15 seconds using a homogenizer. The homogenization speed and time can be adjusted according to the sample type if necessary (eg: harder tissue requires higher speed and longer time). After homogenization, the samples were incubated at -20°C for 10 minutes. After incubation, centrifuge for 10 minutes at 4°C and centrifugal force >10000g. Take the supernatant into a centrifuge tube, and centrifuge each centrifuge tube at low speed, so that the sample droplets on the tube wall sink to the bottom of the centrifuge tube. Take the supernatant and dry it completely with a vacuum c...
Embodiment 2
[0056] Take the plasma sample and centrifuge for 15 minutes at 4°C under the condition of centrifugal force>15000g (12000rpm). After centrifugation, take the supernatant to a 0.5mL centrifuge tube; Add 90 μL of pre-cooled LC-MS or HPLC grade methanol to the centrifuge tube of 30 μL sample, fully vortex the centrifuge tube containing the sample and methanol to completely precipitate the protein, and then centrifuge the centrifuge tube at low speed to make the mixture in the tube sink to bottom of the centrifuge tube. Place the vortexed mixture in a -20°C refrigerator for at least 1 hour; after 1 hour, take the centrifuge tube out of the refrigerator, centrifuge at a centrifugal force >10,000g for 15 minutes, and pipette 90 μL of the supernatant into a new microcentrifuge tube. 90 μL supernatant was completely dried with a vacuum centrifugal concentrator, and the drying was stopped when there was no solvent at the bottom of the centrifuge tube, and the obtained dried sample was ...
Embodiment 3
[0059] Take the urine sample and centrifuge it for 15 minutes at 4°C under the conditions of centrifugal force >15000g (12000rpm), and transfer the supernatant to a 1.5mL centrifuge tube after centrifugation. Use a vacuum centrifugal concentrator to completely dry the obtained supernatant, stop drying when there is no solvent at the bottom of the centrifuge tube, and obtain a dry sample.
[0060] If the urine sample has not been filtered before, the supernatant needs to be filtered through a 0.2-0.3 μm filter. After filtration, take the filtrate into a centrifuge tube. Then use a vacuum centrifugal concentrator to dry, and stop drying when there is no solvent at the bottom of the centrifuge tube to obtain a dry sample.
[0061] For the derivatization method of dried samples and the high-efficiency analysis method of non-targeted metabolomics, see Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com