Application of non-oral berberine
A berberine, non-oral technology, applied in the field of non-oral berberine or its salts, can solve the problems of poor gastrointestinal absorption, buried pharmacological activity, low bioavailability and the like, and achieves less adverse reactions, good effects, The effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
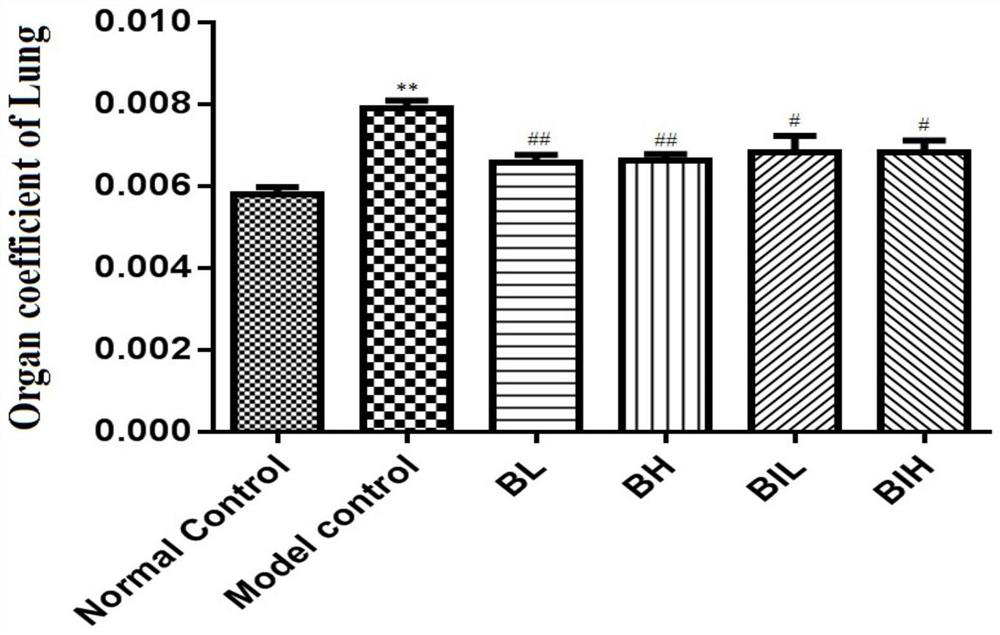
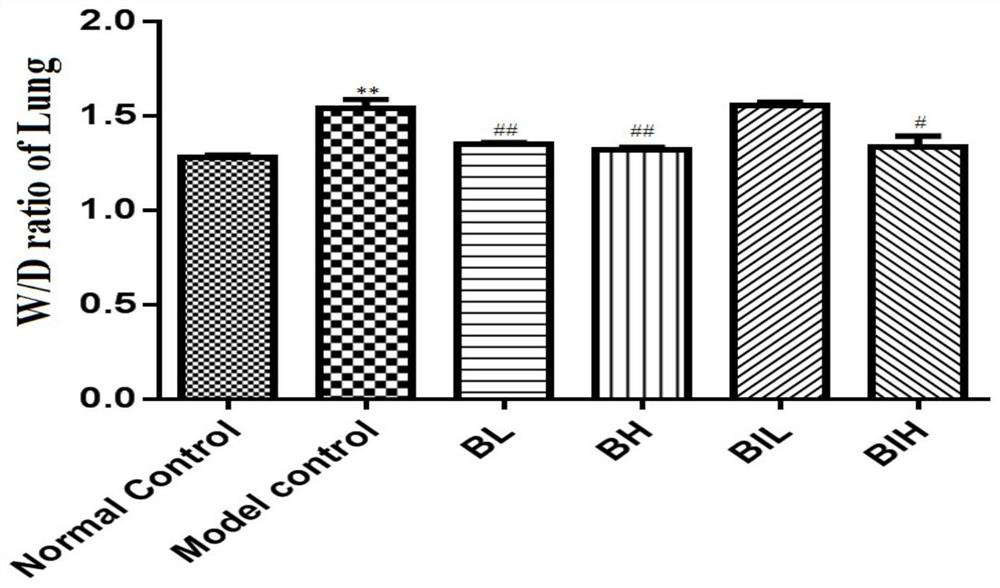
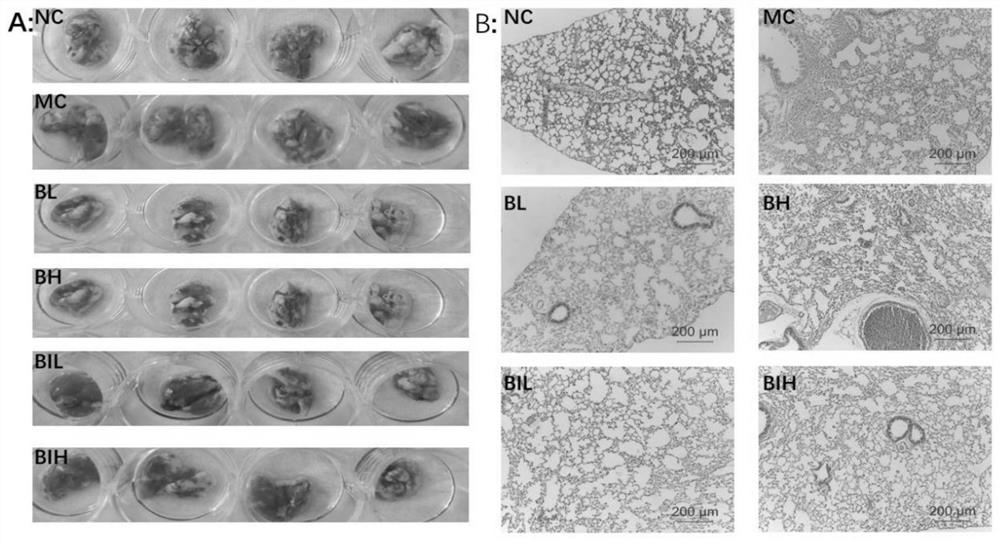
[0038] Example 1 Protective effect of berberine atomized inhalation on lung injury in mouse acute pneumonia model
[0039] C57B / 6J mice (Jiangsu Jicui Experimental Animal Co., Ltd.), male, weighing 30±2g, 32 were randomly divided into normal control group (Normal control, NC), acute pneumonia model group (Model control, MC), berberine Nebulized low-dose inhalation group (Berberine inhaled low dose, BIL) and Berberine inhaled high-dose group (Berberine inhaled high dose, BIH), 8 rats in each group.
[0040] The mouse model of acute pneumonia was replicated as follows: the mice were injected intraperitoneally with 45 mg / kg of pentobarbital sodium according to body weight, and after the righting reflex disappeared, the mouth was gently opened, the tongue was pulled out, and 50 μL of LPS solution was sucked with a pipette ( Prepared according to body weight, the administration dose is 5 mg / kg), injected into the oral cavity through the posterior pharyngeal wall, quickly pinched th...
Embodiment 2
[0046] Example 2 Protective Effects of Subcutaneous Injection of Berberine on Lung Injury in Mouse Acute Pneumonia Model
[0047] C57B / 6J mice (Jiangsu Jicui Experimental Animal Co., Ltd.), male, weighing 30±2g, 32 were randomly divided into normal control group (Normal control, NC), acute pneumonia model group (Model control, MC), berberine Subcutaneous injection low dose group (1mg / kg, Berberine low dose, BL) and berberine subcutaneous injection high dose group (2mg / kg, Berberine high dose, BH), 8 rats in each group.
[0048] The mouse acute pneumonia model replication method is the same as in Example 1.
[0049] The experimental scheme is as follows: After the modeling was completed, the BL group was subcutaneously injected with 1 mg / kg berberine hydrochloride via a 3 mm micro-syringe, and the BH group was given 2 mg / kg berberine hydrochloride via a 3 mm micro-injection subcutaneous injection. After 12 hours of modeling, the body weight of mice in each group was recorded, ...
Embodiment 3
[0054] Example 3 Protective effect of berberine subcutaneous injection and aerosol inhalation on ACE2 receptor-associated pneumonia and lung injury
[0055] The method for replicating the acute pneumonia model in mice, the regimen of administration in groups, and the detection methods for related indicators of sample collection are the same as those in Examples 1 and 2.
[0056] In this example, Western blotting was used to detect the expression of ACE2 protein in lung tissue of mice in each group by subcutaneous injection and aerosol inhalation of berberine. The results of the study showed that subcutaneous injection and aerosol inhalation of berberine could significantly inhibit the expression of ACE2 receptor in lung tissue of acute pneumonia (see Figure 4 ). It is suggested that the non-oral route of subcutaneous injection and aerosol inhalation of berberine may produce effects through acting on ACE2 protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com