Individualized tumor therapeutic vaccine and preparation method thereof
A tumor treatment and tumor technology, applied in the field of biomedicine, can solve the problems of unstable therapeutic effect, hidden safety risks and ineffectiveness of therapeutic vaccines, achieve anti-tumor immune response, high safety and reliability, and inhibit tumor growth. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Identification and synthesis of tumor neoantigen polypeptides
[0061] Surgical collection of tumor tissue and para-cancerous tissue or venous blood samples of patients, the samples are subjected to whole exome and RNA sequencing by next-generation sequencer, and the sequencing data are analyzed by bioinformatics using algorithms (such as NetMHC, NetMHCpan, MHCFlurry) for mutations, mutations Can be caused by missense mutations or insertions / deletions. Translate the mutated gene into a polypeptide sequence, and find out 15-35 polypeptide sequences according to the HLA affinity with the patient; the length of the polypeptide varies from 8-35 amino acids. Polypeptides are synthesized in solid phase in a GMP laboratory, and the purity of the peptides is over 98%. Take 200-800ug of each polypeptide, mix 15-35 polypeptides, and add 1-2mg of adjuvant to prepare a personalized tumor neoantigen vaccine.
Embodiment 2
[0062] Embodiment 2: Preparation of neoantigen vaccine
[0063] Merge 15-35 neoantigen peptides into 4 peptide pools, each of which contains:
[0064] 5-10 peptides, the concentration of each peptide is 200-800μg / ml
[0065] 5%DMSO
[0066]Saline for injection.
[0067] Mix each peptide pool and adjuvant to prepare a personalized neoantigen peptide vaccine, wherein the adjuvant consists of the following components:
[0068] 1-3mg / ml Poly IC
[0069] 1-3mg / ml PolyLC
[0070] 50-200ug / ml STING agonist
Embodiment 3
[0071] Embodiment 3: Neoantigen vaccine animal experiment
[0072] The highly tumorigenic mouse Panc02 cell line was cultured in DMEM medium supplemented with 10% fetal bovine serum, 1% L-glutamine and 0.5% penicillin / streptomycin in 10% CO 2 37°C. Whole WES and RNA sequencing of Panc02 cells were performed to identify immunogenic mutations and predict the affinity of epitope neoantigens to H-2Db and H-2Kb MHC I and MHC II molecules.
[0073] Predicted epitopes 8-35 amino acids in length containing the identified non-synonymous mutations were analyzed for affinity to H-2Db and H-2Kb MHC I molecules.
[0074] Synthesize peptides to prepare neoantigen vaccines.
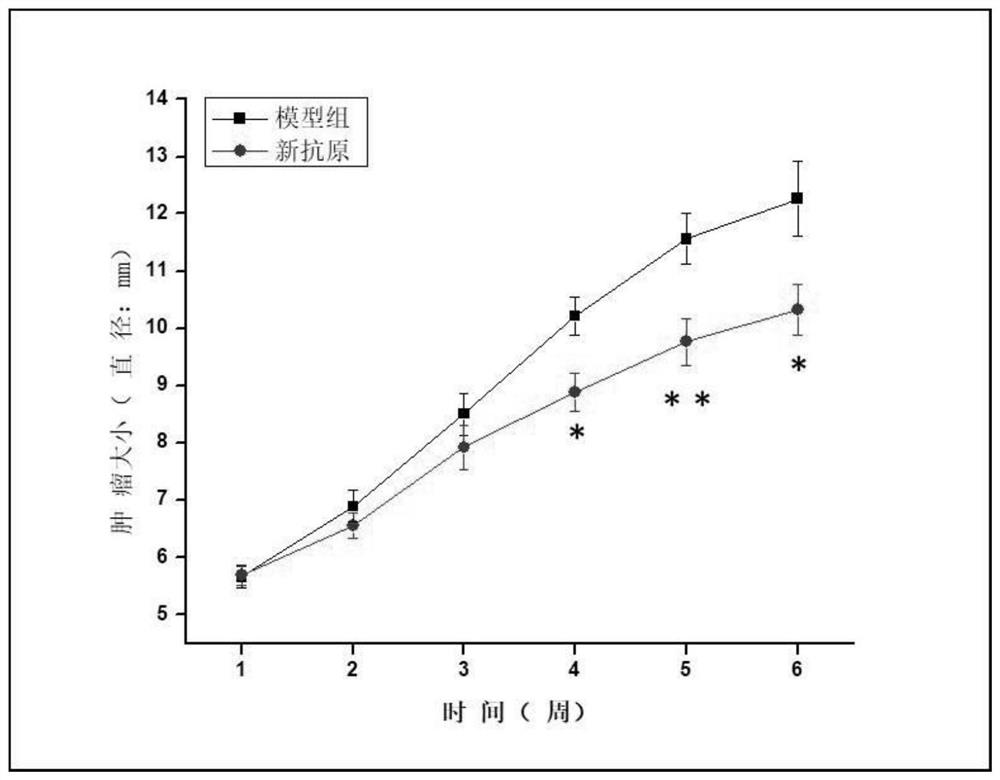
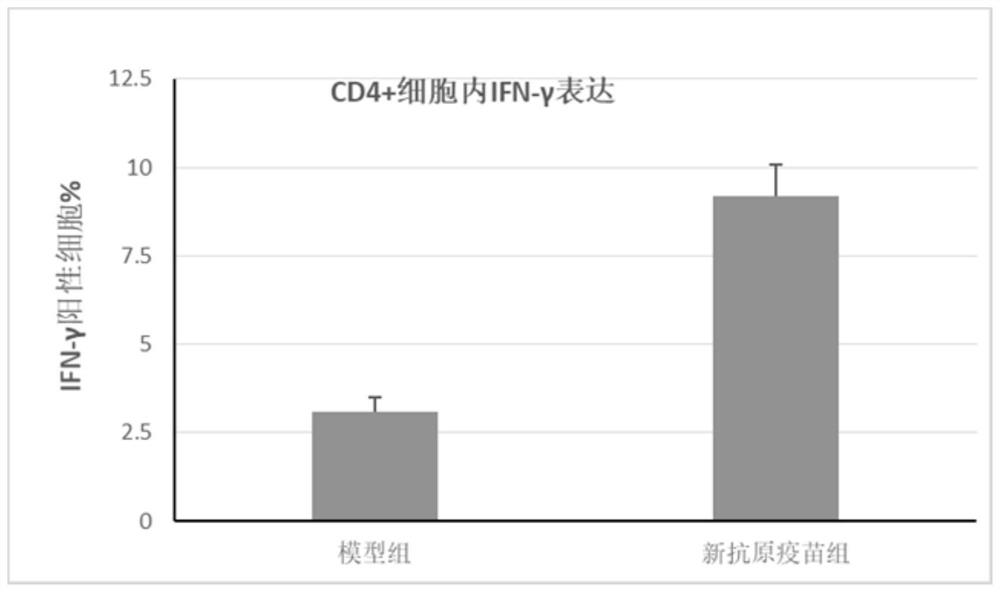
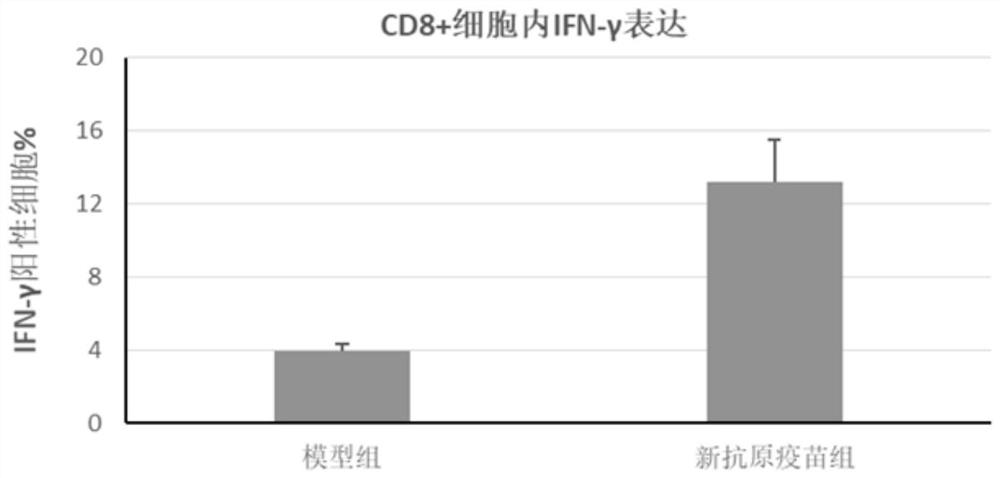
[0075] Each group consisted of 8 C57BL / 6 mice, and 10 6 Panc02 cells were used to establish tumor models. On day 0, day 3, and day 10 after inoculation of tumor cells, mice were injected with neoantigen vaccine, Poly(IC:LC) and STING agonist combined with adjuvant. All mice were raised under pathogen-free conditio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com