Magnetocaloric-immune combined medicine and application thereof
A drug and magnetocaloric technology, applied in the field of combined magnetocaloric-immune drugs, can solve the problems of high side effects of the body and the development limitation of immunocombination therapy, and achieve the effect of good thermal effect, inhibition of remote tumor growth, and high-efficiency tumor treatment effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 A kind of magnetothermic-immune combination therapy medicine
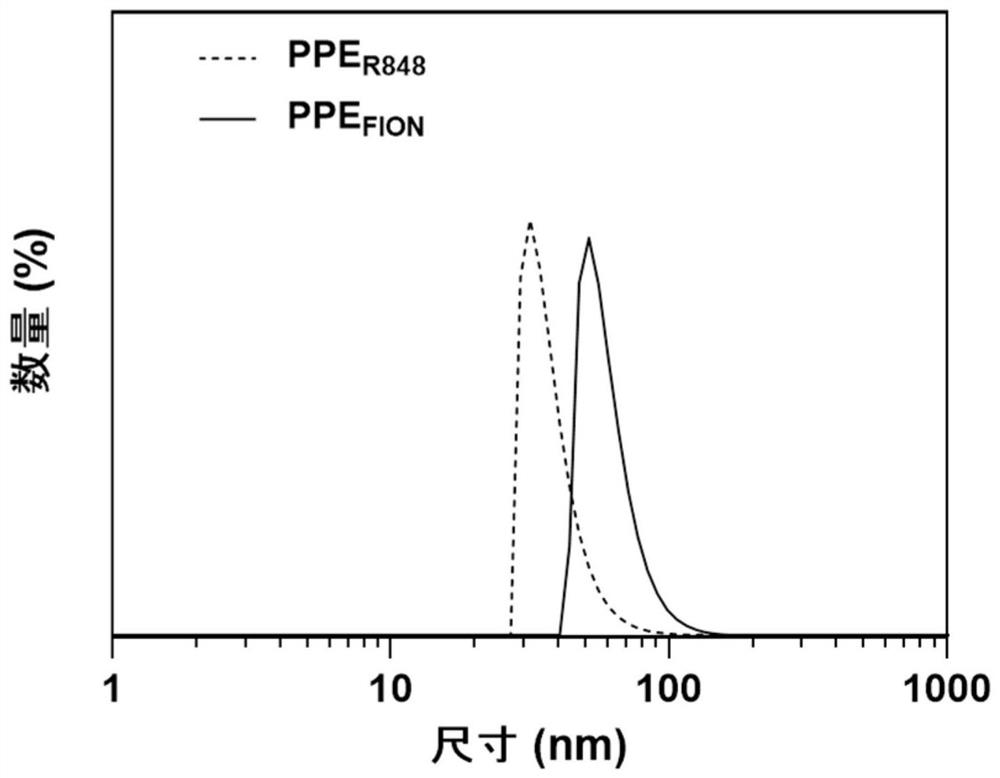
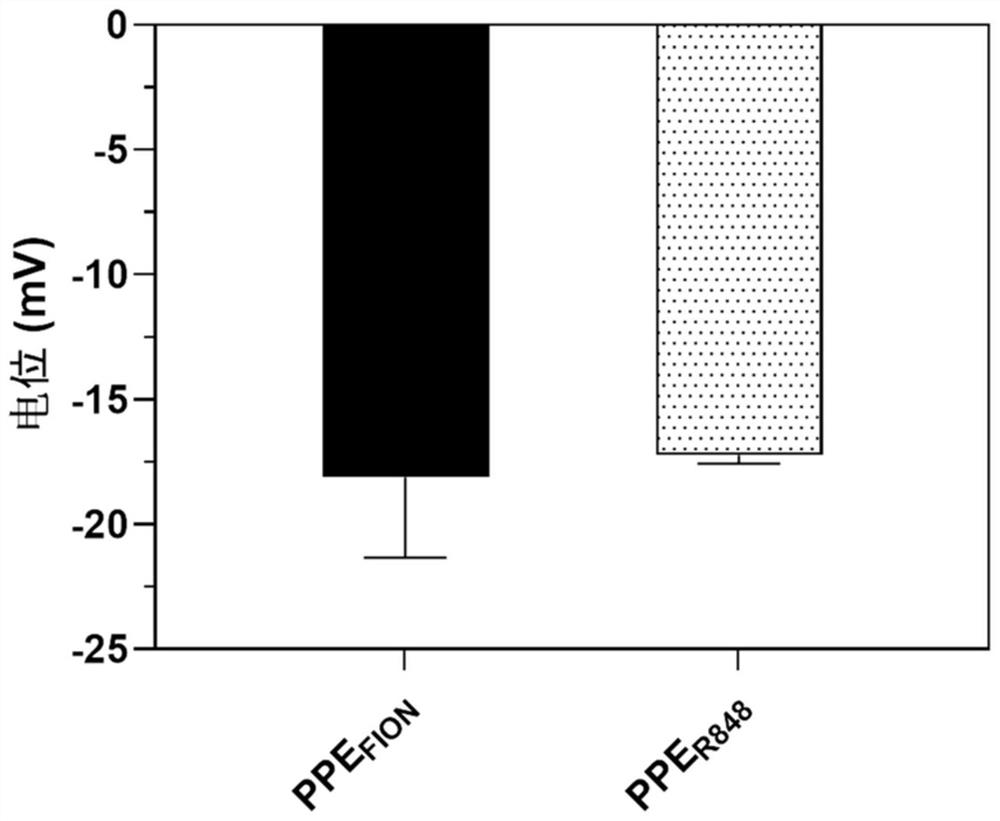
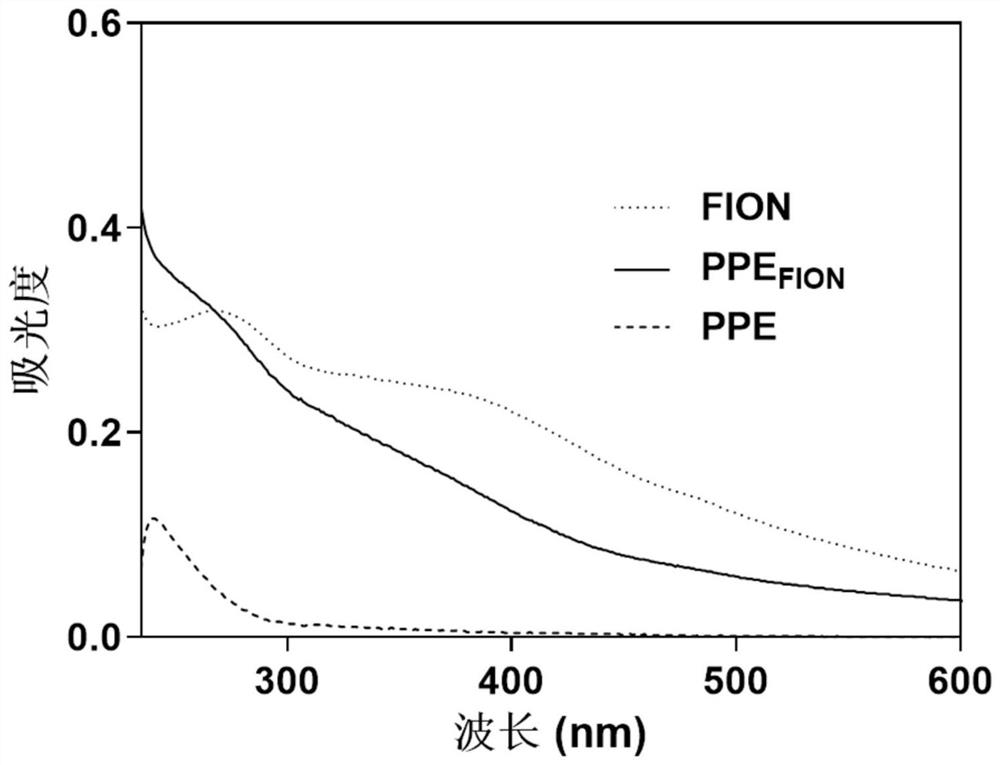
[0059] (1) A combination of magnetocaloric-immune therapy comprising amphiphilic polyphosphate-modified ferromagnetic nanocrystals (PPE FION ) and amphiphilic polyphosphate modified immune adjuvants (PPE R848 ).
[0060] (2) Amphiphilic polyphosphate modified ferromagnetic nanocrystals (PPE FION ) preparation: the ultrasonic self-assembly method was used to prepare amphiphilic polyphosphate-modified ferromagnetic nanocrystals, specifically as follows: Weigh 20 mg polyethylene glycol-b-poly 2-hexyloxy-2-oxygen-1,3 , 2-dioxaphospholane (PEG-PHEP) (polyethylene glycol-b-poly 2-hexyloxy group-2-oxygen-1,3, the synthetic method of 2-dioxaphospholane References: Junxia, Wang, Yang, et al.NIR-Activated Supersensitive Drug Release Using Nanoparticles with a Flow Core[J].Advanced Functional Materials,2016,26(41):7516-7525), and 1mL ferromagnetic nanocrystals (FION ) solution (2mg / mL, the solvent is...
Embodiment 2
[0066] Embodiment 2 A kind of magnetothermic-immune combination therapy medicine
[0067] (1) A combination of magnetocaloric-immune therapy comprising amphiphilic polyphosphate-modified ferromagnetic nanocrystals (PPE FION ) and amphiphilic polyphosphate modified immune adjuvants (PPE R848 ).
[0068] (2) Amphiphilic polyphosphate modified ferromagnetic nanocrystals (PPE FION ) preparation: the ultrasonic self-assembly method was used to prepare amphiphilic polyphosphate-modified ferromagnetic nanocrystals, as follows: Weigh 10 mg polyethylene glycol-b-poly2-hexyloxy-2-oxygen-1,3 , 2-dioxaphospholane (PEG-PHEP) (polyethylene glycol-b-poly 2-hexyloxy group-2-oxygen-1,3, the synthetic method of 2-dioxaphospholane References: Junxia, Wang, Yang, et al.NIR-Activated Supersensitive Drug Release Using Nanoparticles with a Flow Core[J].Advanced Functional Materials,2016,26(41):7516-7525), and 1mL ferromagnetic nanocrystals (FION ) solution (2mg / mL, the solvent is dimethyl sulf...
Embodiment 3
[0074] Embodiment 3 A kind of magnetothermic-immune combination therapy medicine
[0075] (1) A combination of magnetocaloric-immune therapy comprising amphiphilic polyphosphate-modified ferromagnetic nanocrystals (PPE FION) and amphiphilic polyphosphate modified immune adjuvants (PPE R848 ).
[0076] (2) Amphiphilic polyphosphate modified ferromagnetic nanocrystals (PPE FION ) preparation: the ultrasonic self-assembly method was used to prepare amphiphilic polyphosphate-modified ferromagnetic nanocrystals, as follows: Weigh 30 mg of polyethylene glycol-b-poly 2-hexyloxy-2-oxygen-1,3 , 2-dioxaphospholane (PEG-PHEP) (polyethylene glycol-b-poly 2-hexyloxy group-2-oxygen-1,3, the synthetic method of 2-dioxaphospholane References: Junxia, Wang, Yang, et al.NIR-Activated Supersensitive Drug Release Using Nanoparticles with a Flow Core[J].Advanced Functional Materials,2016,26(41):7516-7525), and 1mL ferromagnetic nanocrystals (FION ) solution (2mg / mL, the solvent is dimethyl s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com