Preparation method of sisomicin sulfate sterile powder for injection
A technology of sisomicin sulfate and sterile powder injection, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, powder transportation, etc., can solve the problem of large power consumption of freeze dryers and stable products It can solve the problems of poor performance and high production cost, and achieve the effect of low impurity content, good stability and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 A kind of preparation method of sisomicin sulfate sterile powder injection for injection
[0025] The present embodiment is a preparation method of sisomicin sulfate sterile powder injection for injection, and the specific preparation process includes the following steps performed in sequence:
[0026] Boil water for injection and let it cool for later use;
[0027] Under nitrogen protection, take 500 mL of boiled water for injection, add 1 g of L-cysteine and stir to fully dissolve, use a 0.45 μm microporous membrane to filter to remove insoluble substances and some residual bacterial microorganisms, and cool to 15 ° C ( Recorded as the first cooling), add 100g of sisomicin sulfate and stir to dissolve, use a small amount of aqueous solution containing 0.1mol / L sodium citrate and 0.1mol / L sodium hydroxide to adjust the pH value to 5.5, and maintain the system throughout the process. The temperature is 15°C, then 2g of medicinal activated carbon is added...
Embodiment 2~6
[0029] Embodiment 2~6 Preparation method of sisomicin sulfate sterile powder injection for injection
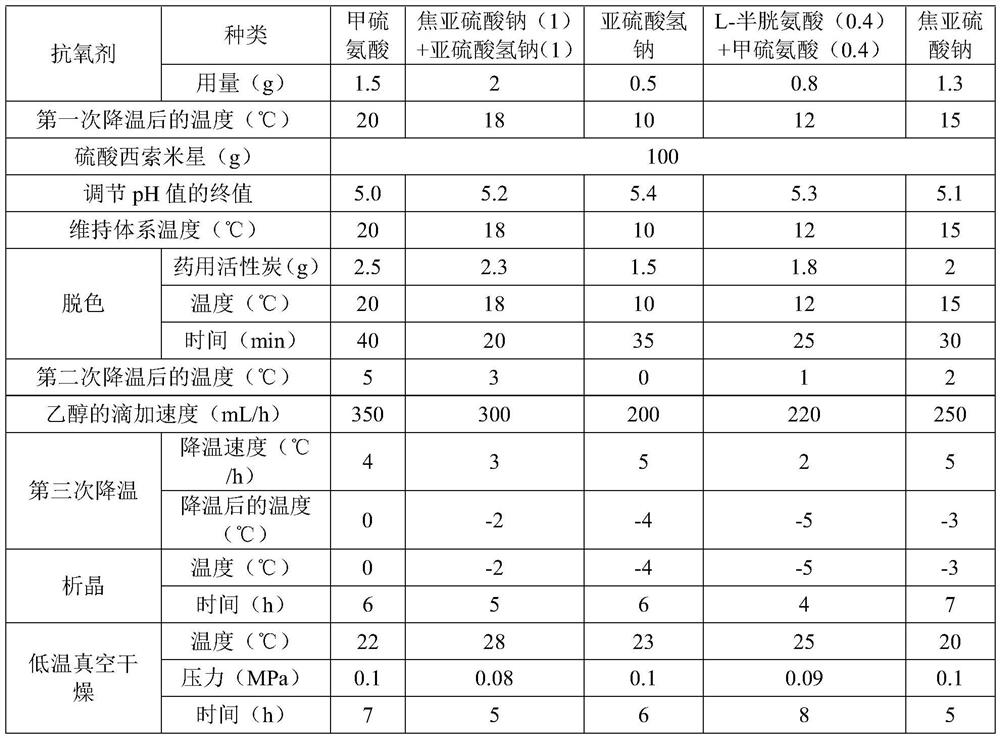
[0030] Embodiments 2-6 are respectively a kind of preparation method of sisomicin sulfate aseptic powder injection for injection, their steps are basically the same as those of embodiment 1, and the difference is only in the amount of raw materials and process parameters. For details, please refer to the details. Table 1:
[0031] List of various process parameters in Table 1 Examples 2 to 6
[0032]
[0033]
[0034] The contents of other parts of Examples 2 to 6 are the same as those of Example 1.
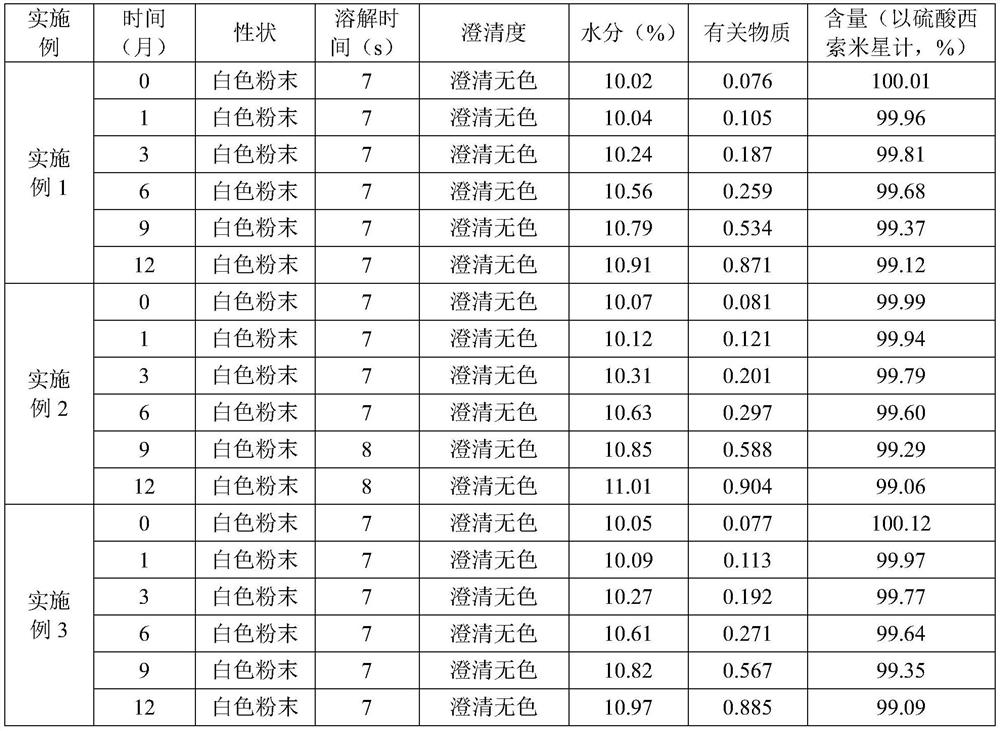
[0035] The sisomicin sulfate sterile powder for injection prepared in Examples 1 to 6 has good stability, high content of the main drug, few impurities, and excellent resolubility.
experiment example 1
[0036] Experimental Example 1 Determination of performance of sisomicin sulfate sterile powder injection for injection
[0037] Comparative Examples 1 to 6 are the comparative tests of the preparation process of sisomicin sulfate sterile powder injection for injection in Example 1 [specification 0.1g (calculated by sisomicin)], and the differences are only as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com