Niazoxanide pharmaceutical composition and preparation method thereof
A technology for nitazoxanide and composition is applied in the field of nitazoxanide pharmaceutical composition and preparation thereof, which can solve the problems of poor fluidity, accelerated degradation of nitazoxanide and the like, and achieves improved stability and simple and easy preparation method. , the effect of reducing the degree of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
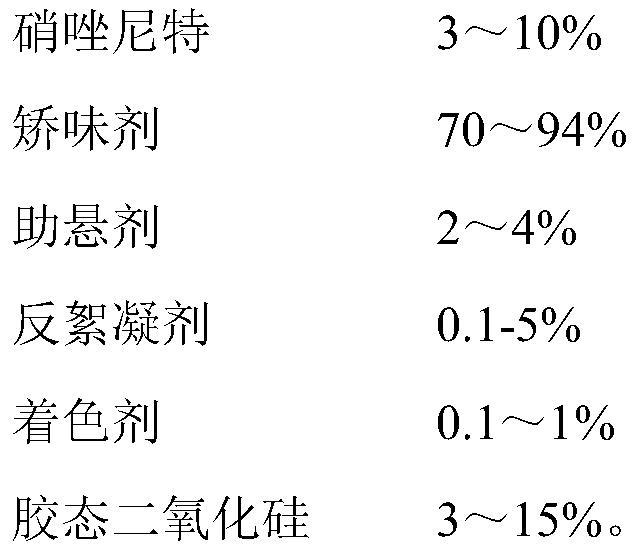
[0039] A pharmaceutical composition of nitazoxanide, made of the following components by weight percentage:
[0040]
[0041] Preparation:
[0042] (1) Mix sucrose, allure red aluminum lake, strawberry powder essence, and colloidal silicon dioxide in a single-column mixer with 5% by weight of the prescription amount to obtain a physical mixture;
[0043] (2) The above physical mixture is mechanically mixed in a pulverizing granulator to achieve the effect of mixing;
[0044] (3) Add nitazoxanide, xanthan gum, anhydrous citric acid, sodium citrate, microcrystalline cellulose-carboxymethyl cellulose sodium and the remaining amount of sucrose into a single-column mixer for mixing, and mix evenly. A dry suspension of nitazoxanide was obtained.
Embodiment 2
[0046] A pharmaceutical composition of nitazoxanide, made of the following components by weight percentage:
[0047]
[0048] Preparation:
[0049] (1) Mix sucrose, allure red aluminum lake, strawberry powder essence, and colloidal silicon dioxide in a single-column mixer with 5% by weight of the prescription amount to obtain a physical mixture;
[0050] (2) The above physical mixture is mechanically mixed in a pulverizing granulator to achieve the effect of mixing;
[0051] (3) Add nitazoxanide, xanthan gum, anhydrous citric acid, sodium citrate, microcrystalline cellulose-carboxymethyl cellulose sodium and the remaining amount of sucrose into a single-column mixer for mixing, and mix evenly. A dry suspension of nitazoxanide was obtained.
Embodiment 3
[0053] A pharmaceutical composition of nitazoxanide, made of the following components by weight percentage:
[0054]
[0055]
[0056] Preparation:
[0057] (1) Mix sucrose, allure red aluminum lake, strawberry powder essence, and colloidal silicon dioxide in a single-column mixer with 5% by weight of the prescription amount to obtain a physical mixture;
[0058] (2) The above physical mixture is mechanically mixed in a pulverizing granulator to achieve the effect of mixing;
[0059] (3) Add nitazoxanide, xanthan gum, anhydrous citric acid, sodium citrate, microcrystalline cellulose-carboxymethyl cellulose sodium and the remaining amount of sucrose into a single-column mixer for mixing, and mix evenly. A dry suspension of nitazoxanide was obtained.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap