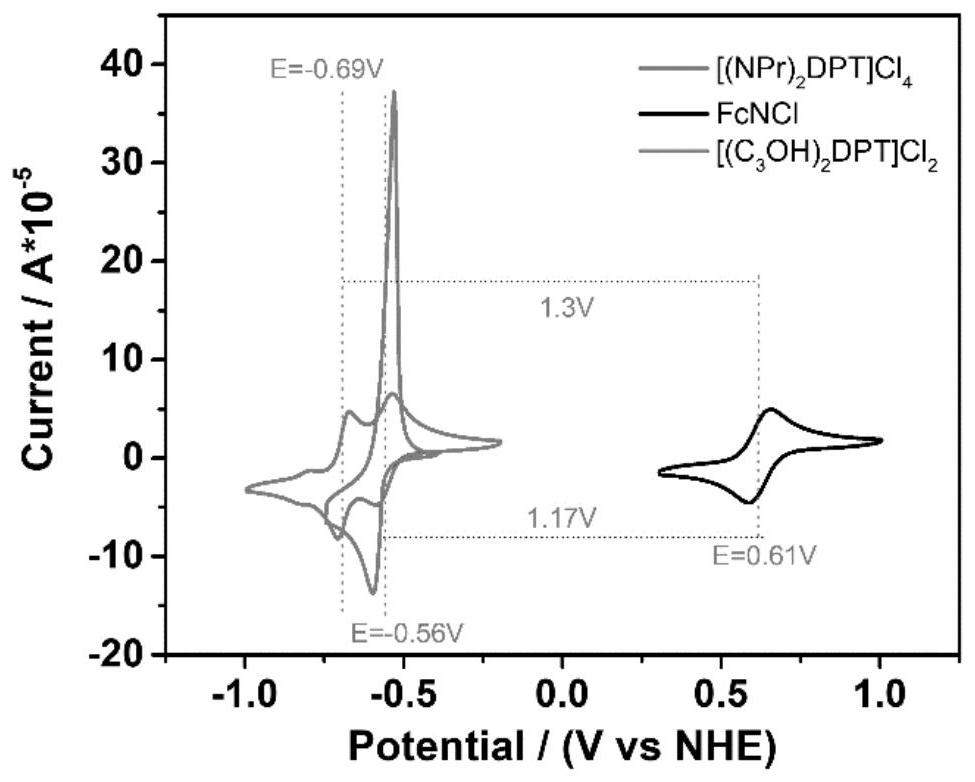
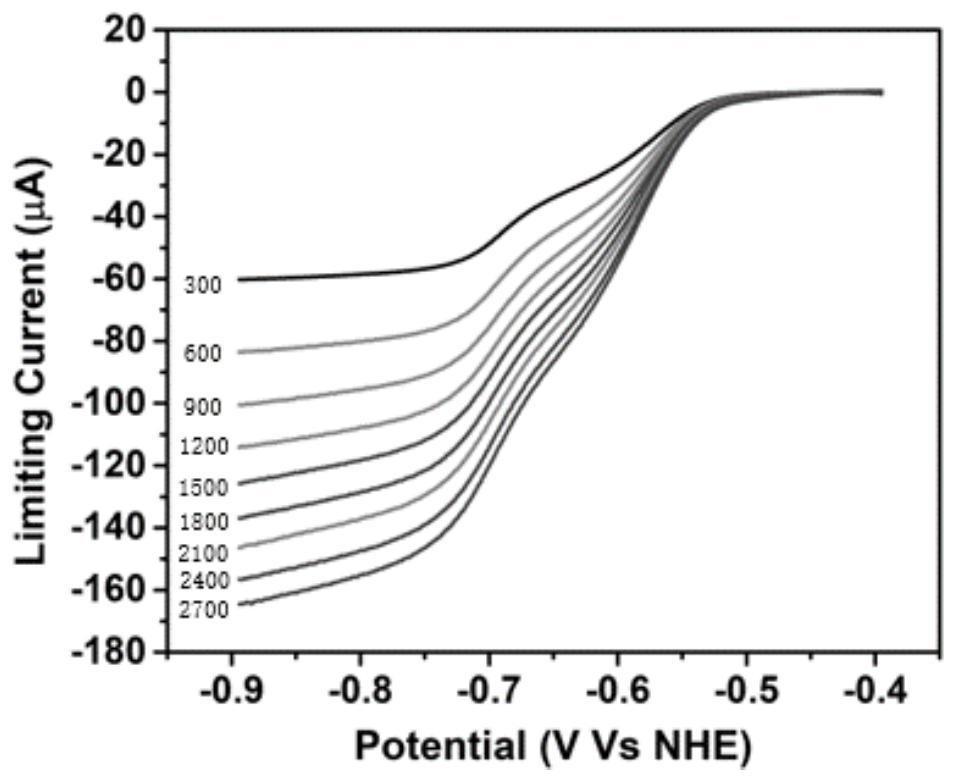
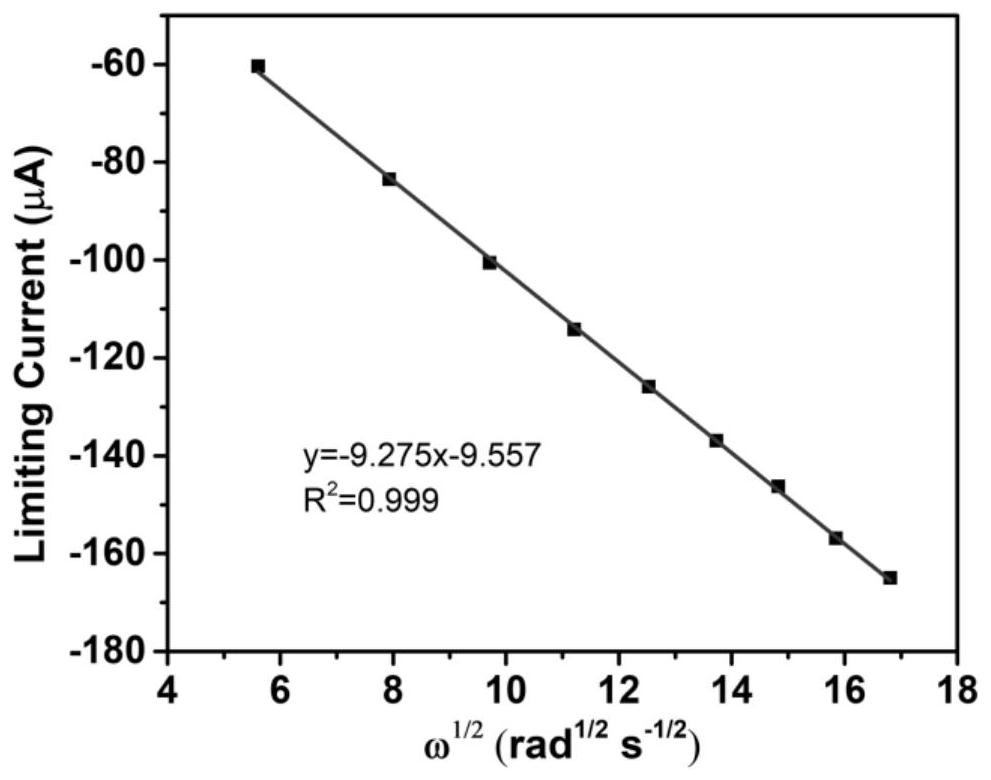
Thienyl and selenophenyl viologen derivatives, and synthesis method and application thereof
A synthetic method, viologen technology, applied in electrochemical generators, regenerative fuel cells, fuel cells, etc., can solve the problems of low electron transfer rate, low solubility, low conjugation, etc., and achieve outstanding kinetic performance, Improve solubility and conductivity, and effect of charge balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] 1, the synthetic method of a class of thienyl viologen derivatives of the present invention comprises the following steps:
[0067] 1): Precursor A of thienyl viologen derivatives synthesized by Suzuki coupling reaction;
[0068] Under an argon protective atmosphere, take 2,5-dibromothiophene (4.13mmol), 4-pyridine borate (10.33mmol), and tetrakistriphenylphosphine palladium (0.21mmol) in a pressure-resistant tube. Phenylphosphine palladium is used as a reaction catalyst, and the molar ratio of the three is 1:2.5:0.05. Add methyl trioctyl ammonium chloride (phase transfer catalyst), 40mL oxygen-free potassium carbonate solution (2M) and 60mL freshly steamed toluene (anhydrous and oxygen-free toluene as solvent), the volume ratio of potassium carbonate solution and toluene is 2:3. Stir well and heat the reaction at 130°C for 3d.
[0069] Post-treatment process: Cool the reaction mixture to room temperature, take the organic matter in the upper layer after liquid separa...
Embodiment 1
[0096] 1, the synthetic method of a class of thienyl viologen derivatives of the present invention comprises the following steps:
[0097] 1): Precursor A of thienyl viologen derivatives synthesized by Suzuki coupling reaction;
[0098] Take a 100mL branch bottle and a 250mL pressure tube to dry, and use a pump to cool it for later use. Prepare 40 mL of potassium carbonate solution (2M) and bubble for 20 minutes. Take 1g of 2,5-dibromothiophene (4.13mmol), 2.12g of 4-pyridine borate (10.33mmol), the molar ratio of the two is 1:2.5, add it to the 250mL pressure tube prepared in advance, and put it in the glove box Weigh 260 mg tetrakistriphenylphosphine palladium (0.21 mmol) in it as a reaction catalyst. Under argon atmosphere, 1 mL of methyltrioctylammonium chloride (phase transfer catalyst), 40 mL of potassium carbonate solution (2M), 60 mL of freshly distilled toluene were added. Stir well and heat the reaction at 130°C for 3d.
[0099] Post-treatment process: Cool the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com