Method for producing trans-4-Hyp (trans-4-hydroxy-L-proline) through transformation of recombinant escherichia coli
A proline, proline hydroxylase technology, applied in the field of recombinant Escherichia coli transformation to produce trans-4-hydroxy-L-proline, can solve the problems of poor stability, low catalytic efficiency and the like, and achieves conversion rate High, high utilization rate of raw materials, easy to control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
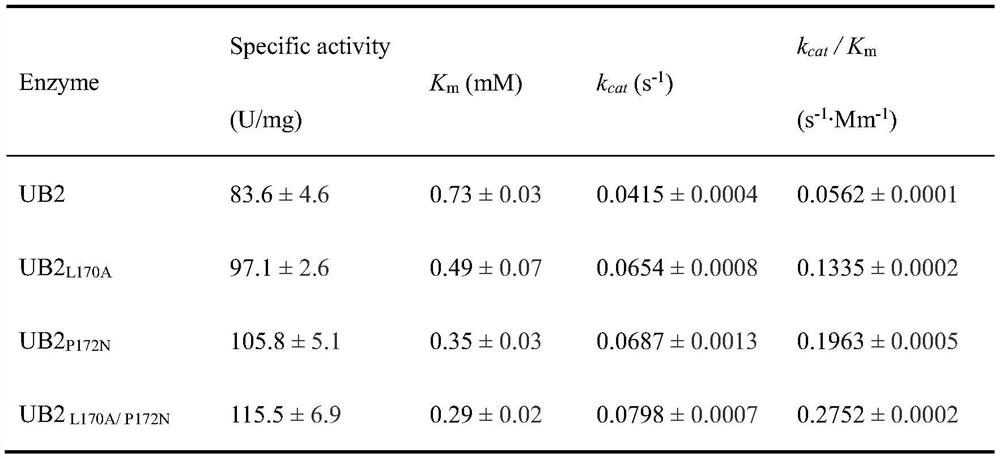
[0031] Embodiment 1: the construction of mutant
[0032] Construction of recombinant strains of E.coli BL21 / pET28a-UB2 and its mutants
[0033] 1. Synthesis and transformation of the full sequence of the P4H enzyme gene
[0034] According to the uncultured bacteria esnapd2 (GeneID: AGS49339.1) P4H sequence published by GENBANK, the codon was optimized to obtain the gene encoding L-proline hydroxylase whose sequence is shown in SEQ ID NO.1. The target gene and expression plasmid pET28a were digested with restriction endonuclease EcoR I / Hind III, and the recombinant plasmid pET28a-UB2 was obtained after the target gene was connected with the vector. The recombinant plasmid pET28a-UB2 was transformed into Escherichia coli E.coli BL21 by chemical transformation method, the colony grown on LB and ampicillin pressure plate was picked, cultured in shake flask, and the plasmid was extracted for single and double enzyme digestion verification to obtain Recombinant bacteria E.coli BL2...
Embodiment 2
[0050] Example 2: Detection of whole cell transformation performance of recombinant strains
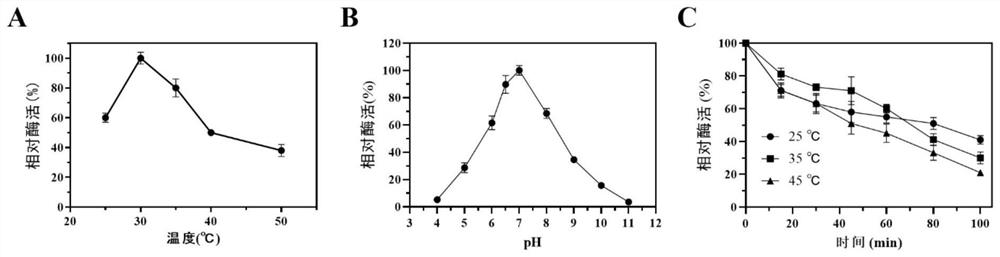
[0051] The recombinant bacteria E.coli BL21 / pET28a-UB2 and its mutant recombinant strains constructed in Example 1 were activated and transferred into the self-inducing medium, and the cells were collected for whole cell transformation. The transformation system was 10 mL, and the temperature was 40 ℃, the conversion pH is 7.0, cell wall permeabilization agent Triton 10 μL, add 20 mM L-proline, 20 mM α-ketoglutarate, 1 mM FeSO 4 -7H 2 O and 5 mM ascorbic acid for transformation. The product Hyp in the transformation solution was detected by HPLC. The product Hyp was detected, indicating that the recombinant Escherichia coli strain with transformed L-proline into Hyp was successfully constructed.
[0052] Blank control: The plasmid pET28a was transformed into E.coli BL21 for expression to obtain the recombinant strain E.coliBL21 / pET28a. Pick the colonies grown on LB and kanamycin p...
Embodiment 3
[0053] Embodiment 3: biotransformation method produces trans-4-hydroxyl-L-proline
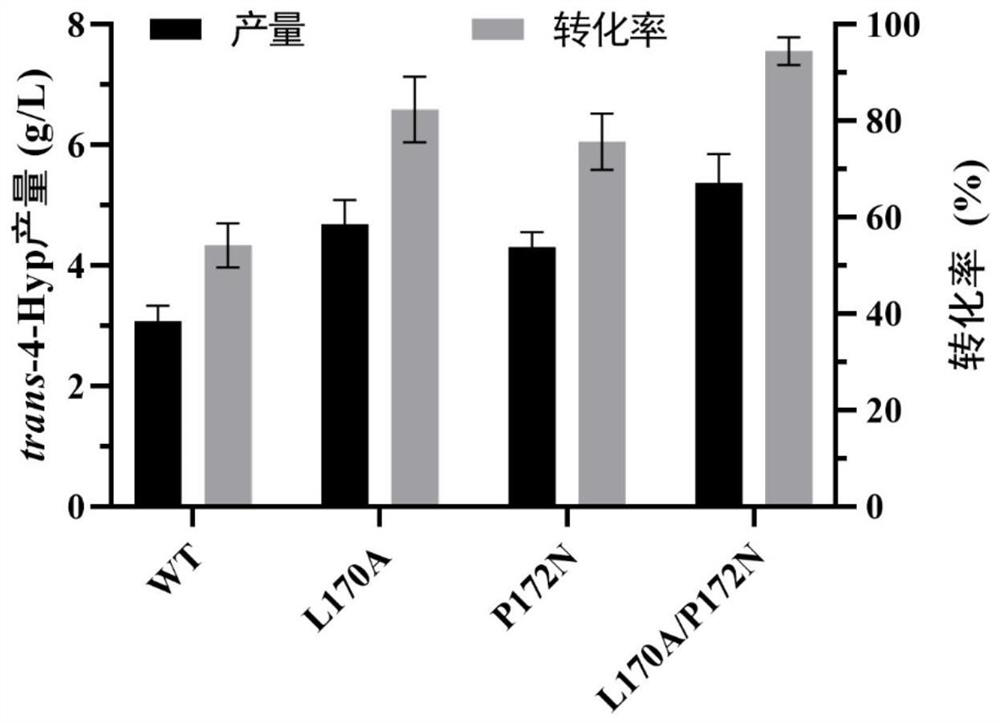
[0054] The recombinant bacteria E.coli BL21 / pET28a-UB2 and its mutant recombinant strains constructed in Example 1 were inoculated as a single colony in 10 mL of LB medium, and cultured for 12 hours. Inoculate 10% of the inoculum into a 1L baffled Erlenmeyer flask filled with 250mL autoinduction medium, and culture at 25°C for about 24h. Collect the bacteria, and use 100mL, 100mM, pH 7.0 Na 2 HPO 4 -Resuspend the recombinant E. coli cells in citric acid buffer to a wet weight of 250g / L, add 100 μL of the cell wall permeabilizer Triton, add 40mML-proline, 40mMα-ketoglutarate, 1mM FeSO 4 -7H 2 0, 5mM ascorbic acid for transformation, the transformation temperature is 30°C, and the transformation pH is 7.0. After 24 hours of whole-cell transformation, the Hyp in the whole-cell transformation solution of the recombinant bacteria was determined by HPLC. After 24 hours of whole cell transformati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com