Preparation method of modified polyvinylidene fluoride in lithium ion battery
A polyvinylidene fluoride and lithium-ion battery technology, applied in secondary batteries, battery electrodes, circuits, etc., can solve the problems of discharge specific capacity attenuation, weak van der Waals force, and electrode particles are easy to fall off, so as to improve the wetting effect and increase Adhesive effect, the effect of enhancing the adhesive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
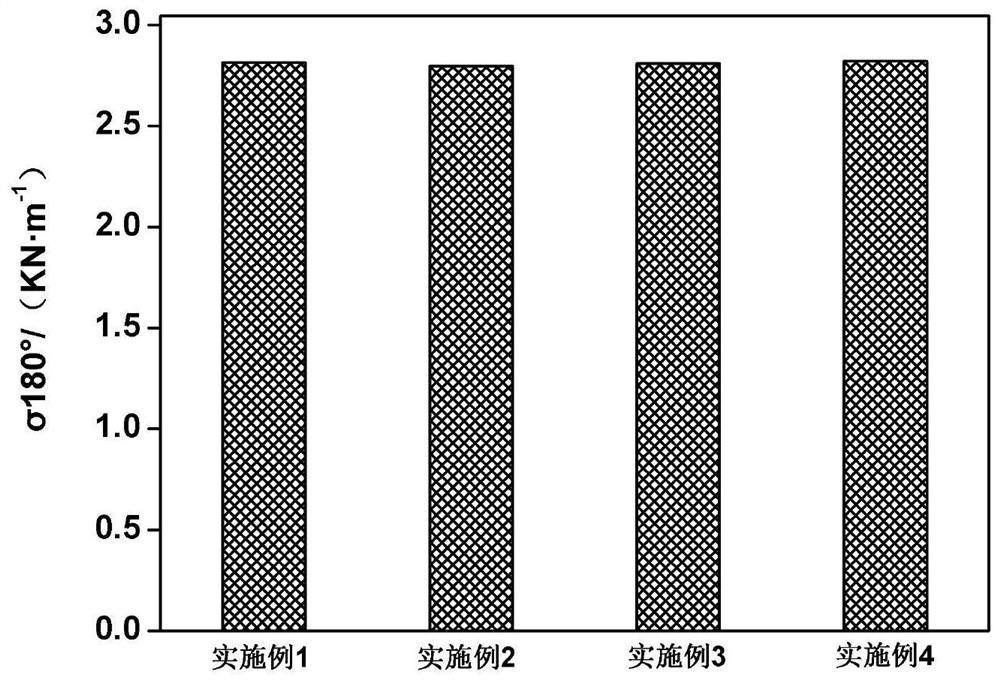
Examples
Embodiment 1
[0016] A preparation method for modified polyvinylidene fluoride in lithium ion batteries, specifically comprising the following steps:
[0017] S1: Add graphene oxide into N,N-dimethylformamide, ultrasonically disperse, then add itaconic acid and azobisisobutyronitrile, heat to 60°C under nitrogen atmosphere, and react with magnetic stirring for 6h. After cooling, filter and dry; wherein the mass ratio of graphene oxide, itaconic acid and azobisisobutyronitrile is 1:0.46:0.24.
[0018] S2: Add the modified graphene oxide obtained in step S1 to N,N-dimethylacetamide, then sonicate for 5 hours, then add tin dichloride to raise the temperature to 60°C and stir for 2 hours, then add polyvinylidene fluoride and polyvinylpyrrolidone, continue to magnetically stir at this temperature for 10h, then cool, put into a ball mill after vacuum drying, and grind for 2h to obtain the binder; wherein modified graphene oxide, N,N-dimethylacetamide The mass-volume ratio of neutralized tin dich...
Embodiment 2
[0020] A preparation method for modified polyvinylidene fluoride in lithium ion batteries, specifically comprising the following steps:
[0021] S1: Add graphene oxide into N,N-dimethylformamide, ultrasonically disperse, then add itaconic acid and azobisisobutyronitrile, heat to 70°C under nitrogen atmosphere, and react with magnetic stirring for 10h. After cooling, filter and dry; wherein the mass ratio of graphene oxide, itaconic acid and azobisisobutyronitrile is 2:0.66:0.39.
[0022] S2: Add the modified graphene oxide obtained in step S1 to N,N-dimethylacetamide, then sonicate for 8 hours, then add tin dichloride to raise the temperature to 70°C and stir for 3 hours, then add polyvinylidene fluoride and polyvinylpyrrolidone, continue to magnetically stir at this temperature for 16 hours, then cool, put into a ball mill after vacuum drying, and grind for 3 hours to obtain the binder; wherein modified graphene oxide, N,N-dimethylacetamide The mass-volume ratio of neutraliz...
Embodiment 3
[0024] A preparation method for modified polyvinylidene fluoride in lithium ion batteries, specifically comprising the following steps:
[0025] S1: Add graphene oxide into N,N-dimethylformamide, ultrasonically disperse, then add itaconic acid and azobisisobutyronitrile, heat to 65°C under nitrogen atmosphere, and react with magnetic stirring for 8h. After cooling, filter and dry; wherein the mass ratio of graphene oxide, itaconic acid and azobisisobutyronitrile is 1.3:0.54:0.29.
[0026] S2: Add the modified graphene oxide obtained in step S1 to N,N-dimethylacetamide, then sonicate for 7 hours, then add tin dichloride to raise the temperature to 65°C and stir for 2.5 hours, then add polyvinylidene fluoride Ethylene and polyvinylpyrrolidone, continue to magnetically stir at this temperature for 12 hours, then cool, put into a ball mill after vacuum drying, and grind for 2.5 hours to obtain the binder; wherein modified graphene oxide, N,N-dimethyl The mass volume ratio of acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com