Pneumocystis carinii recombinant protein as well as preparation method and application thereof
A technology of recombinant protein and spore bacteria, which is applied in the medical field, can solve the problems of clinical toxic side effects and increase of clinical drug resistance reports, and achieve the effect of simple detection method, good cost and cost control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] A kind of Pneumocystis carinii recombinant protein His-A12 of the present embodiment 1-85 , whose amino acid sequence is shown in SEQ ID NO: 1; encoding the Pneumocystis carinii recombinant protein His-A12 1-85 The nucleotide sequence of is shown in SEQ ID NO:2.
Embodiment 2
[0082] Pneumocystis carinii recombinant protein His-A12 in a kind of embodiment 1 of the present embodiment 1-85 The preparation method, preparation steps are as follows:
[0083] 1. Establishment of Pneumocystis japonicum animal model and DNA extraction
[0084] (1) Establishment of animal models:
[0085] Take 30 Kunming female rats of clean grade and raise them in open cages for 1 week. After adapting to the environment, they are divided into two cages, A and B. Wherein, 20 mice in cage A were subcutaneously injected with dexamethasone sodium phosphate injection in the groin of mice, each of 0.5 mg was injected once every three days, and administered for 8 weeks in total, during which the drinking water (containing 1g / L Tetracycline (cold boiled water to prevent bacterial infection) and litter, and normal pelleted feed; a total of 10 mice in cage B were subcutaneously injected with phosphate buffer saline (PBS) solution in the groin as a control.
[0086] During the expe...
Embodiment 3
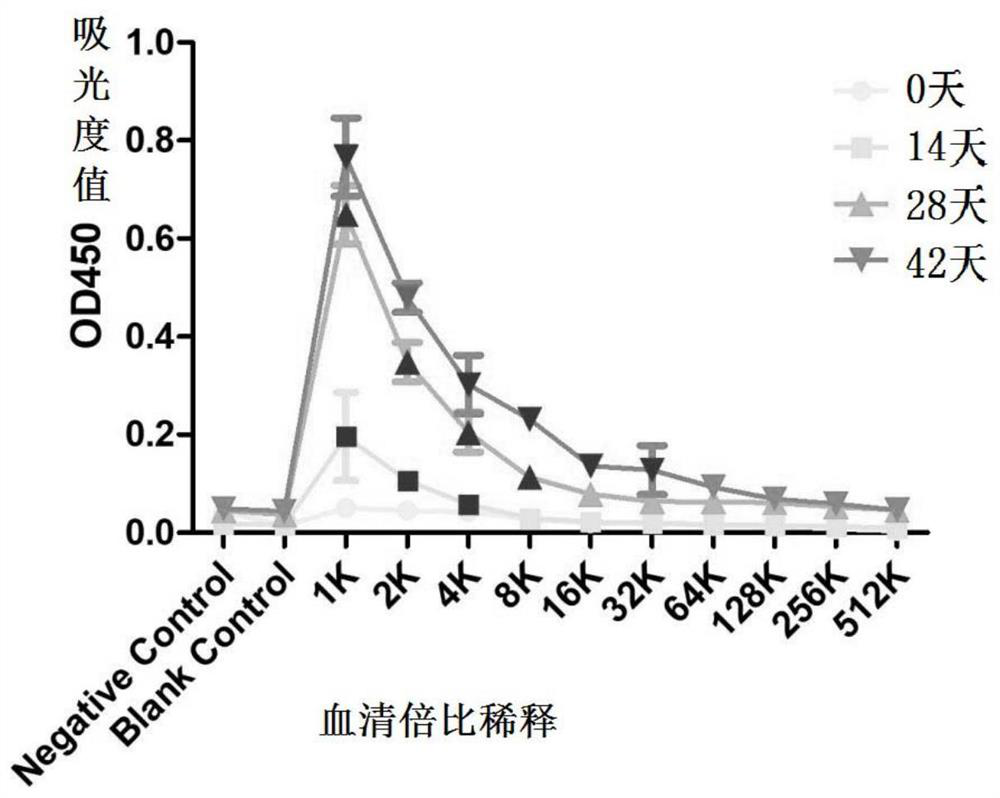
[0177] This example is used to illustrate the recombinant protein His-A12 in the above examples 1-85 The immunization procedure of mice and its immune protection effect.
[0178] 1. Immunization program
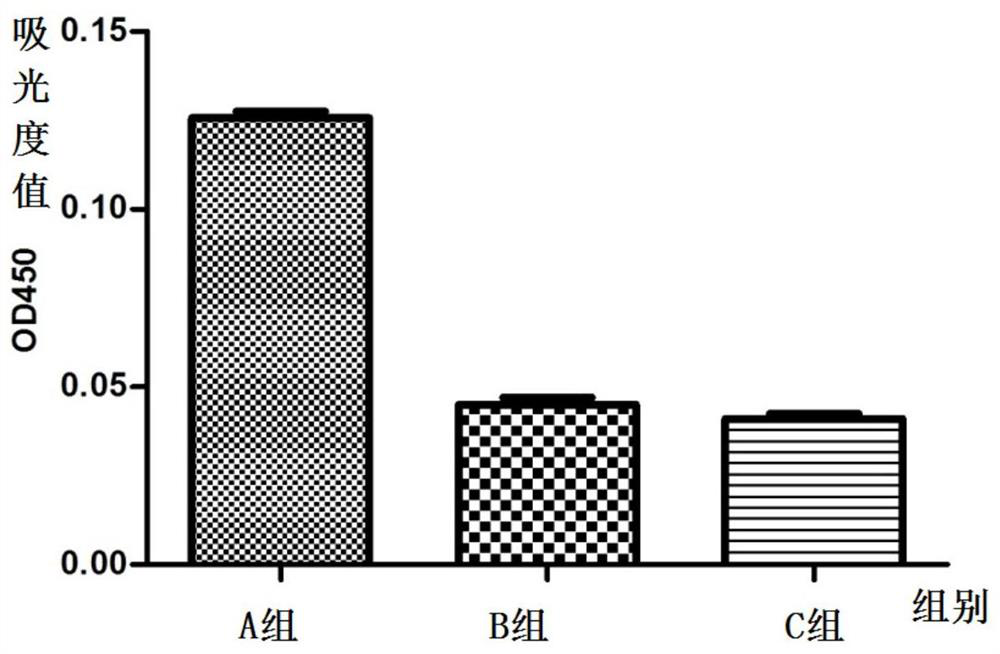
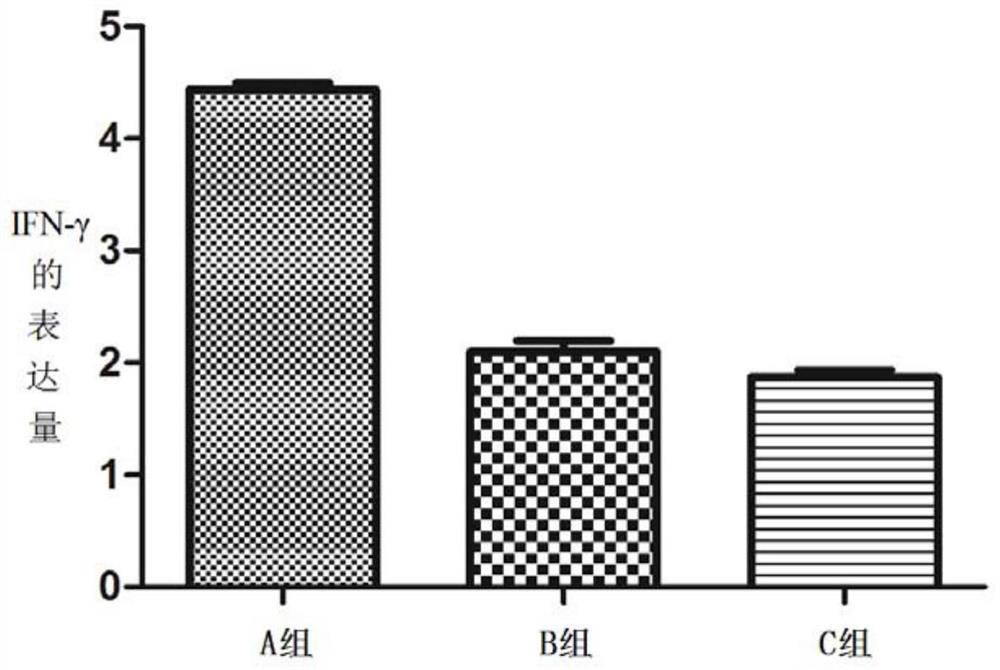
[0179] (1) Animal selection and grouping: 45 Kunming female mice were randomly divided into three cages, A, B, and C, with 15 mice in each cage. The mice in the three cages were all exposed to the external environment, and they drank unlimited high-pressure sterilized water during the feeding process, and the mouse bedding and food were replaced regularly during the feeding process.
[0180] (2) Immunization scheme: His-A12 1-85 Protein antigen immunization;
[0181] Dosage: 150μg / piece;
[0182] Group A: immune to His-A12 1-85 protein antigen;
[0183] Group B: immune PBS+adjuvant;
[0184] Group C blank control.
[0185] On day 0, before immunization, the sera of mice in three cages were collected by docking their tails as negative controls. Group A will emulsify c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com