Iron phosphate precursor as well as preparation method and application thereof
An iron phosphate and precursor technology, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of low tap density of iron phosphate precursors, difficult crushing procedures, and reduced production efficiency, etc. The effect of low cost, good processing performance and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of the iron phosphate precursor of the present embodiment comprises the following steps:
[0041] S1. Select ferric nitrate and phosphoric acid as the iron source and phosphorus source respectively. According to the molar ratio of iron in the iron source and phosphorus in phosphoric acid as 0.965:1, add phosphoric acid to ferric nitrate, adjust the pH to 0 with sulfuric acid, and configure Fe 3+ Liquid metal with a concentration of 50g / L;
[0042] S2. Add 50L of molten metal into the reaction kettle, raise the temperature to 90°C at 400r / min, and react for 10h to obtain a slurry;
[0043] S3, filtering the slurry to obtain a filter residue, and then repeatedly washing the filter residue 3 times with pure water to obtain a washed filter residue;
[0044] S4. Dry the obtained filter residue at 100°C. The drying process needs to be turned several times to obtain the iron phosphate dihydrate precursor.
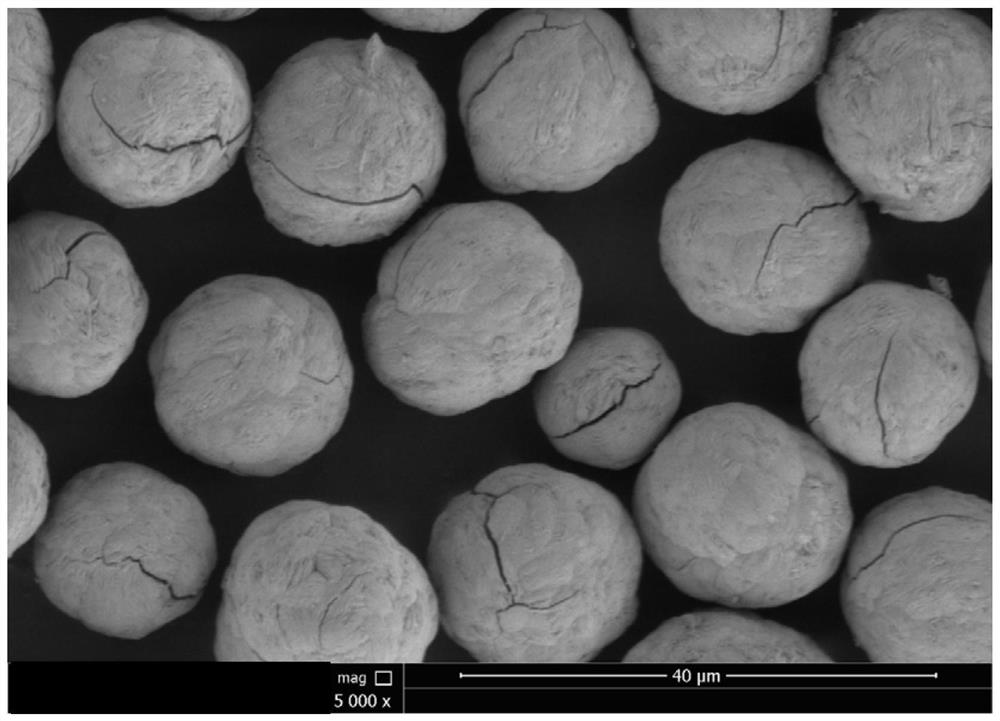
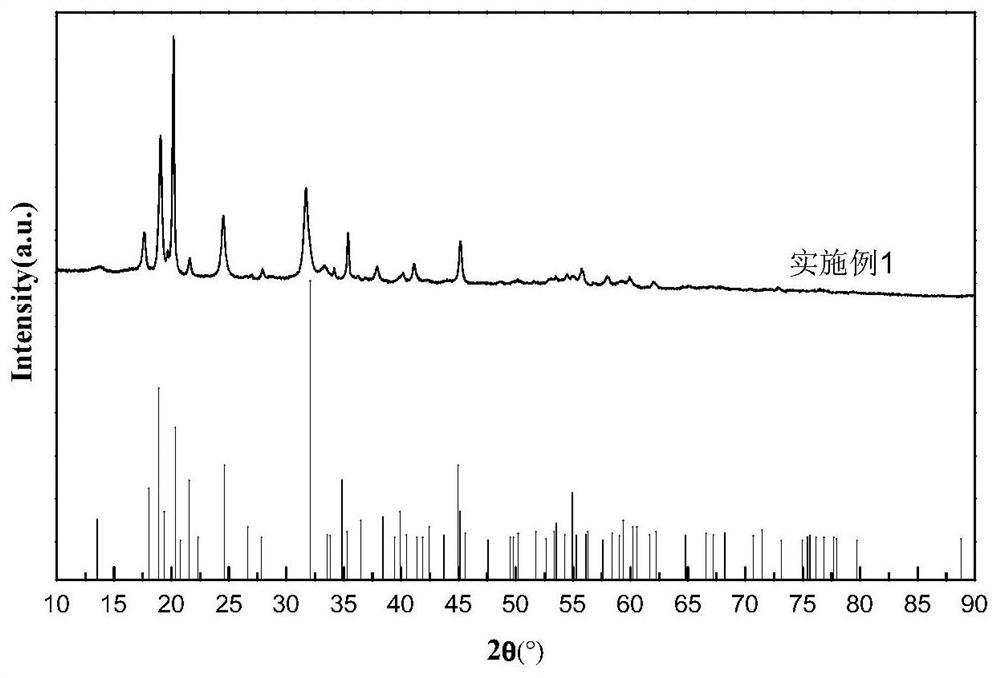
[0045] The physicochemical result of embodime...
Embodiment 2
[0049] The preparation method of the iron phosphate precursor of the present embodiment comprises the following steps:
[0050] S1. Select ferric nitrate and phosphoric acid as the iron source and phosphorus source respectively. According to the molar ratio of iron in the iron source and phosphorus in phosphoric acid as 0.965:1, add phosphoric acid to ferric nitrate, adjust the pH to 0 with sulfuric acid, and configure Fe 3+ Liquid metal with a concentration of 50g / L;
[0051] S2. Add 50L of molten metal into the reaction kettle, raise the temperature to 85°C at 400r / min, and react for 15h to obtain a slurry;
[0052] S3, filtering the slurry to obtain a filter residue, and then repeatedly washing the filter residue 3 times with pure water to obtain a washed filter residue;
[0053] S4. Dry the obtained filter residue at 100°C. The drying process needs to be turned several times to obtain the iron phosphate dihydrate precursor.
Embodiment 3
[0055] The preparation method of the iron phosphate precursor of the present embodiment comprises the following steps:
[0056]S1. Select ferrous sulfate and phosphoric acid as the iron source and phosphorus source respectively. According to the molar ratio of iron and phosphoric acid elements of 0.97:1, add phosphoric acid to ferric nitrate and hydrogen peroxide, adjust the pH to 0.5 with sulfuric acid, and configure Fe 3+ Liquid metal with a concentration of 56g / L;
[0057] S2. Add 50L of molten metal into the reaction kettle, raise the temperature to 90°C at 400r / min, and react for 12h to obtain a slurry;
[0058] S3, filtering the slurry to obtain a filter residue, and then repeatedly washing the filter residue 3 times with pure water to obtain a washed filter residue;
[0059] S4. Dry the obtained filter residue at 100°C. The drying process needs to be turned several times to obtain the iron phosphate dihydrate precursor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com