Bifunctional ionic liquid, preparation method thereof and application of bifunctional ionic liquid in catalytic synthesis of bisphenol compounds
An ionic liquid, dual-function technology, applied in the field of ionic liquid applications, to achieve the effects of increasing productivity, simplifying the use process, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
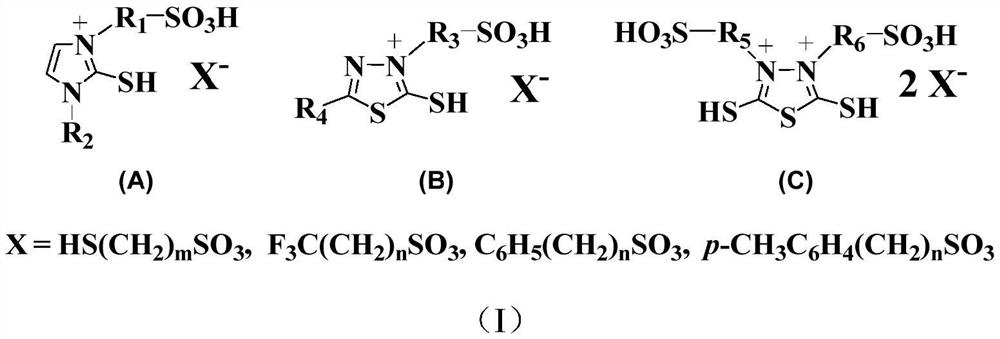
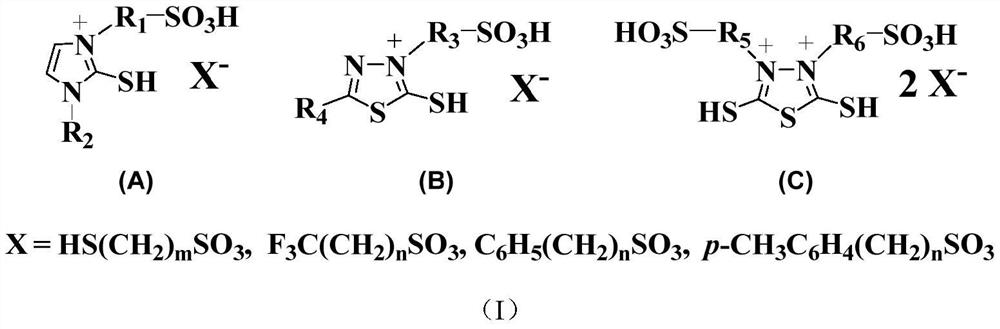
[0049] Accurately weigh 0.1mol (11.7g) of 1-methyl-2-mercaptoimidazole into a 250mL round bottom flask, then add 70mL of absolute ethanol to the flask, heat up to 60°C, and stir to completely dissolve the solid. Slowly add 0.1mol (12.2g) of 1,3-propane sultone dropwise. After the dropwise addition, continue to stir and reflux at 60°C for 8 hours. Washed three times respectively and dried in vacuum to obtain 21.2 g of 3-(sulfopropyl)-1-methyl-2-mercaptoimidazole with a yield of 89%.
[0050] Add 0.05 mol (11.8 g) of the above-prepared 3-(sulfopropyl)-1-methyl-2-mercaptoimidazole and 40 mL of deionized water into a 100 mL round bottom flask in turn, and stir to completely dissolve the zwitterions; Slowly add 0.05mol (8.6g) of p-toluenesulfonic acid dropwise. After the dropwise addition, the temperature is raised to 80° C. for 6 hours. The water was distilled off under reduced pressure, washed three times with ether, and dried in vacuo to obtain 3-(sulfopropyl)-1-methyl-2-mercap...
Embodiment 2
[0052] Accurately weigh 0.1mol (13.2g) of 2-methyl-5-mercapto-1,3,4-thiadiazole in a 250ml round bottom flask, add 125ml of ethyl acetate into the flask, heat up to 95°C, and stir to The solid dissolved completely. Slowly add 0.1mol (13.6g) of 1,4-butane sultone dropwise. After the dropwise addition, continue to stir and reflux at 90°C for 12 hours. The cake was washed three times and dried in vacuum to obtain 18.8 g of 3-(sulfobutyl)-5-methyl-2-mercapto-1,3,4-thiadiazole with a yield of 70%.
[0053] Add 0.05mol (13.4g) of 3-(sulfobutyl)-5-methyl-2-mercapto-1,3,4-thiadiazole prepared above and 40mL deionized water into a 100mL round bottom flask in sequence , stir to completely dissolve the zwitterions; slowly add 0.05mol (7.8g) of 3-mercaptopropanesulfonic acid dropwise, and after the dropwise addition, raise the temperature to 60°C for 12h. The water was distilled off under reduced pressure, washed three times with ether, and dried in vacuo to obtain a bifunctional ionic ...
Embodiment 3
[0055] Accurately weigh 0.1mol (15.0g) of 2,5-dimercapto-1,3,4-thiadiazole into a 250ml round bottom flask, add 120ml of ethyl acetate into the flask, heat up to 95°C, and stir to make the solid completely dissolve. Slowly add 0.2mol (24.4g) of 1,3-propane sultone dropwise. After the dropwise addition, continue to stir and reflux at 95°C for 12 hours. Washed three times respectively and dried in vacuum to obtain 3,4-di(sulfopropyl)-2,5-dimercapto-1,3,4-thiadiazole, 25.6g, yield 65%
[0056] Add 0.05mol (19.7g) of 3,4-bis(sulfopropyl)-2,5-dimercapto-1,3,4-thiadiazole prepared above and 40mL of deionized water into a 100mL round-bottomed In the flask, stir to completely dissolve the zwitterions; slowly add 0.1 mol (15.8 g) of benzenesulfonic acid dropwise, and after the dropwise addition, raise the temperature to 90° C. for 10 h. Distilled off water under reduced pressure, washed three times with ether, and dried in vacuo to obtain 3,4-di(sulfopropyl)-2,5-dimercapto-1,3,4-thia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com