Nitrogen-doped cuprous oxide electrocatalyst, preparation method, electrode and application
A technology of cuprous oxide and electrocatalyst, which is applied in the field of electrocatalysis, can solve the problems of Cu instability and hinder the application of Cu2O, and achieve the effect of improving reaction activity and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] According to a first aspect of the present invention, a method of preparing a nitrogen-doped oxide copper electrocatalyst includes the following steps:
[0026] Water-soluble copper salt and urea are placed in two different porcelain boats, and then placed in a high temperature furnace to 300 to 400 ° C, preferably the temperature rise rate is set to 4 ~ 6 ° C · min -1 And keep this temperature for at least 2 hours, the entire reaction process into flowing argon, the rate is 1 ~ 3L · h -1 After the reaction is completed, it is cooled to room temperature, and the product of the reaction is cleaned with an aqueous solution of alcohol or the like, and finally drying 50 to 70 ° C in a vacuum drying tank, and finally obtaining nitrogen-doped oxide (N-Cu). 2 O).
[0027] In a preferred embodiment, the quality ratio of the water-soluble copper salt and urea is (2 to 4): 5.
[0028] In the second aspect, the nitrogen doped oxide copper electrocatalyst prepared by the preparation me...
Embodiment 1
[0034] Put 300 mg of copper and 0.5 g of urea in two different porcelain boats, and then placed in a tube furnace to 300 ° C, and the temperature rise rate is set to 5 ° C · min -1 And keep this temperature for 2 hours, the process of the entire reaction through the flow of argon, the rate is 2 l · h -1 . After the reaction was completed, the reaction was cooled to room temperature, and the product of the reaction was washed with an aqueous solution of alcohol, and finally drying at 60 ° C in a vacuum drying tank, and finally obtained nitrogen doped oxide (N-Cu) 2 O).
experiment example 1
[0036] Experimental Example 1 Micro Structure Analysis
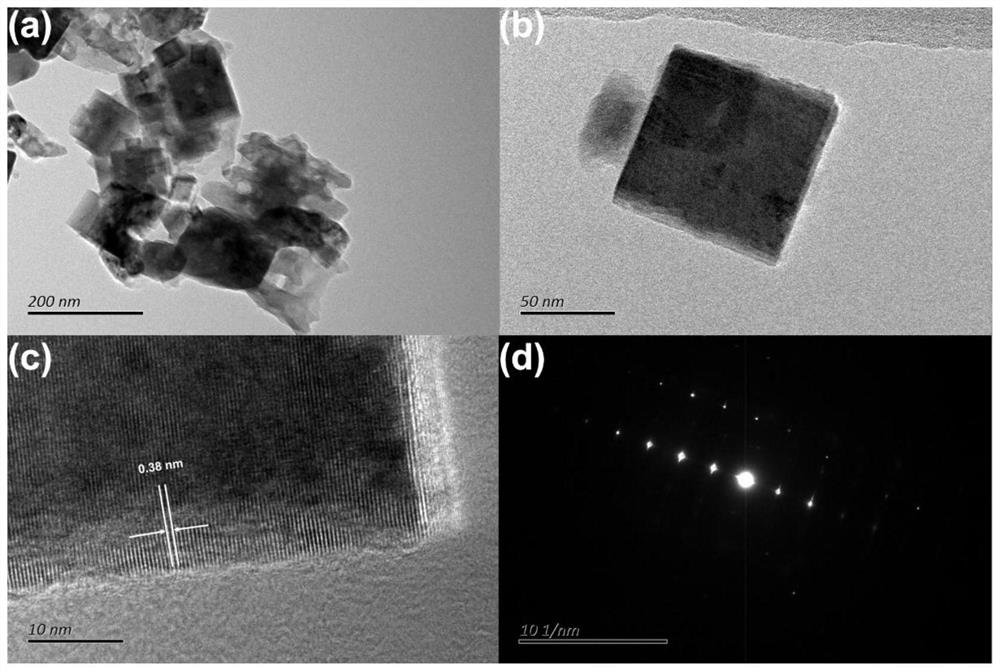
[0037] Embodiment 1 Preparation of nitrogen doped oxide of copper, such as figure 1 Indicated. The overall morphology is a cube structure that is uniform in size ( figure 1 A and 1B). We enlarge the local area and found that the lattice spacing of the crystal is about 0.38 nm to 0.40 nm ( figure 1 c), this is more normal Cu 2 O The lattice spacing is small, and the selected area electronic diffraction (SAED) graphic also shows a high crystallinity ( figure 1 d).
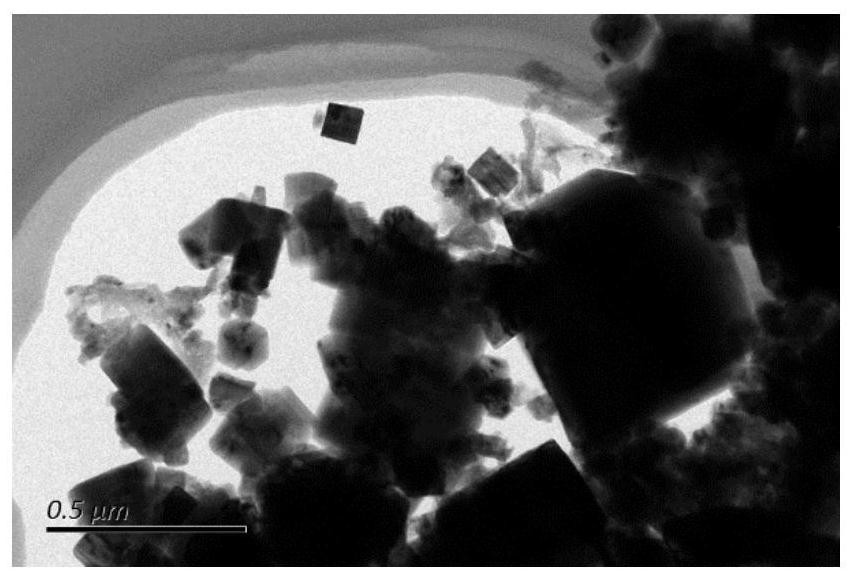
[0038] As contrasting us from electron microscopy figure 2 It can be seen in that there is no nitrogen-doped oxide, agglomerate stack is more serious, and it is basically in the form of agglomeration. It can therefore be determined that the introduction of the nitrogen element changes some lattice structure of the material to rearrange the combination.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com