Application of compound and analogue thereof as ROR gamma regulator
A technology of compounds and analogs, applied in the field of medicine, can solve the problems of unclear target signaling pathways, difficult to distinguish beneficial and harmful components of active monomers, affecting development and use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
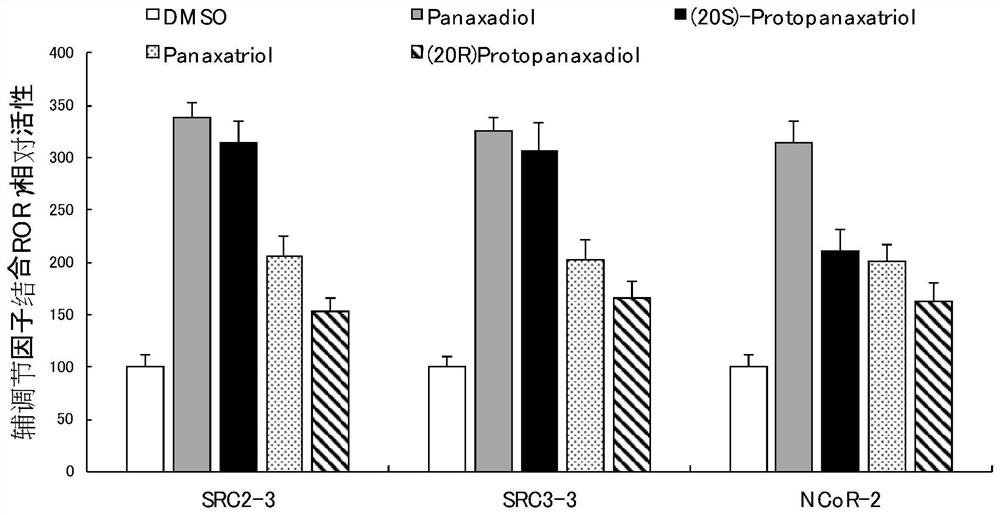
[0066] Example 1 proves that Panaxadiol or its analogs can effectively regulate RORγ recruitment co-regulators, and is a regulator of RORγ.
[0067] figure 1 In order to use the AlphaScreen biochemical method, the final concentration of 1 μM Panaxadiol (Panaxadiol) or its analogues was determined to promote the interaction between coactivator (SRC2-3 and SRC3-3) and co-repressor factor (NCoR-2) motifs and RORγ . The analogs are panaxatriol, protopanaxadiol ((20R)-Protopanaxadiol) and protopanaxatriol ((20S)-Protopanaxatriol). All experiments were performed independently in triplicate. The coregulator polypeptide sequence with an N-terminal biotinylation tag is:
[0068] SRC2-3: QEPVSPKKKENALLRYLLDKDDTKD;
[0069] SRC3-3: PDAASKHKQLSELLRGGSG;
[0070] NCoR-2: GHSFADPASNLGLEDIIRKALMGSF.
[0071] Protein purification: the human RORγLBD (amino acid residue number 262-507) gene was constructed in the pET24a vector containing the 6 polyhistidine fusion tag of Novagen Company....
Embodiment 2
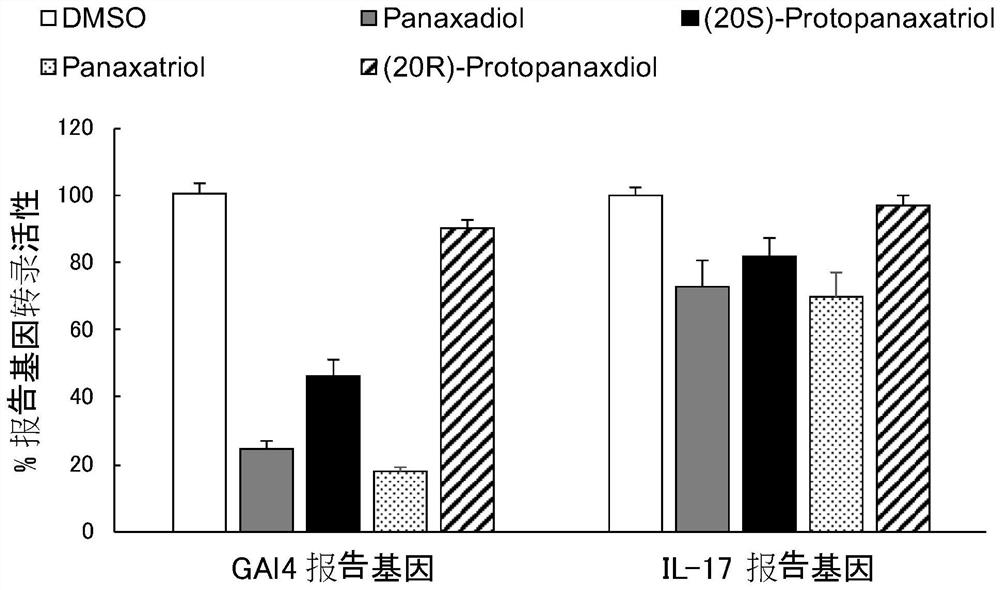
[0077] Example 2 proves that Panaxadiol (Panaxadiol) and its analogues effectively inhibit the transcriptional activation of the RORγ reporter gene, and are inhibitors of RORγ at the cellular level.
[0078] AlphaScreen technology shows that at the biochemical level, panaxadiol and its analogs can simultaneously promote the recruitment of various coactivators and co-inhibitors by RORγ, and the combined effect at the cellular level ultimately affects the activation or inhibition of the compound on the expression of RORγ target genes. We used two general methods of Gal4 reporter gene and IL-17 reporter gene to detect the activation or inhibition of RORγ target gene expression by intracellular compounds, respectively. The Gal4 reporter gene is mainly used to determine the extent to which RORγ regulators regulate the overall transcriptional activity of RORγ target genes, while the IL-17 reporter gene is mainly used to measure the regulation of transcriptional activity of a specific...
Embodiment 3
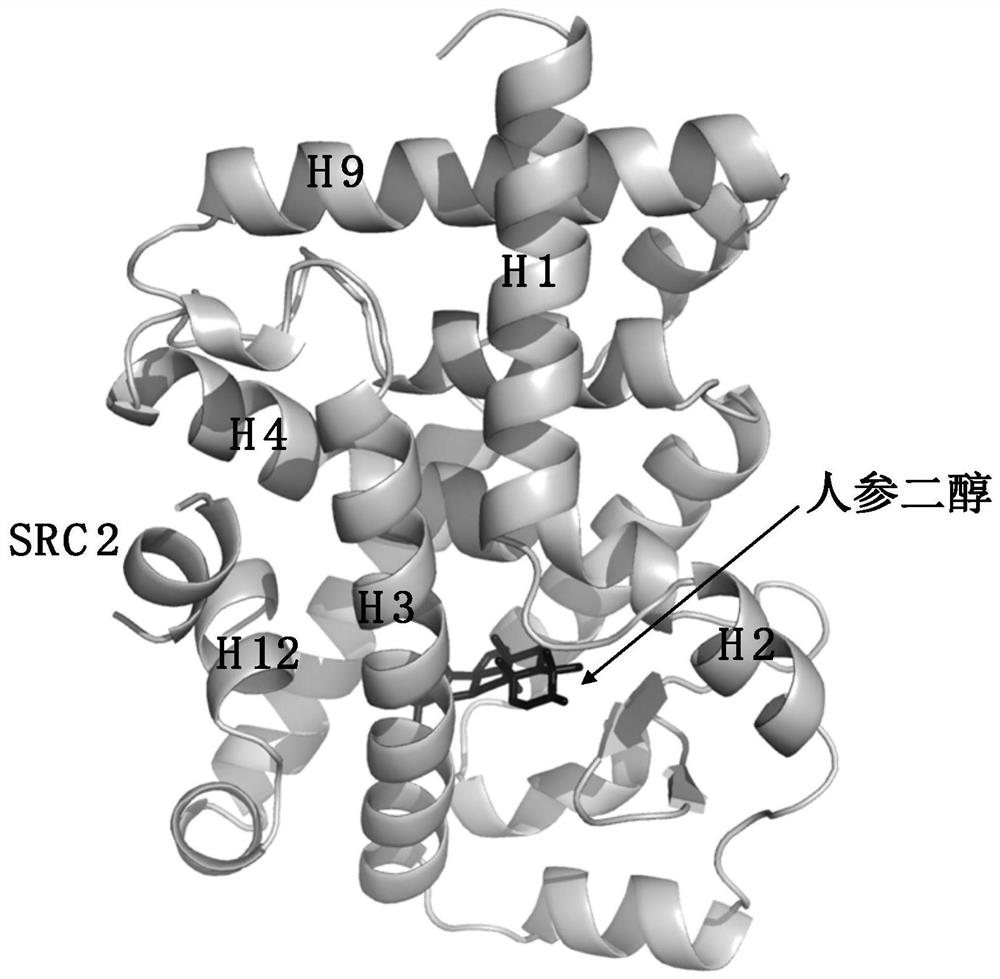
[0080] Example 3 demonstrates the analysis of the crystal structure of the complex of Panaxadiol (Panaxadiol) and RORγ.
[0081] In order to reveal the molecular mechanism of mutual recognition and binding of panaxadiol and RORγ from the atomic level, the crystal structure of the complex formed by panaxadiol and RORγ was analyzed by conventional methods ( image 3 ). Obtain RORγ protein as in Example 1, add panaxadiol 5 times the amount of purified RORγLBD protein, and coactivator SRC2-2 polypeptide (KHKILHRLLQDSS) 2 times the amount of RORγLBD protein, and incubate on ice for 1 h Then, concentrate to 10mg / mL. The crystallization screening kit from Hampton Company was used for crystallization, and the sample complex was mixed with the screening buffer 1:1 and screened by hanging drop crystallization. The crystal grows best at room temperature for about 48 hours, and the best crystallization buffer conditions are: 0.1M BIS-TRIS pH 5.5, 3.0M Sodium chloride. The crystals were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com