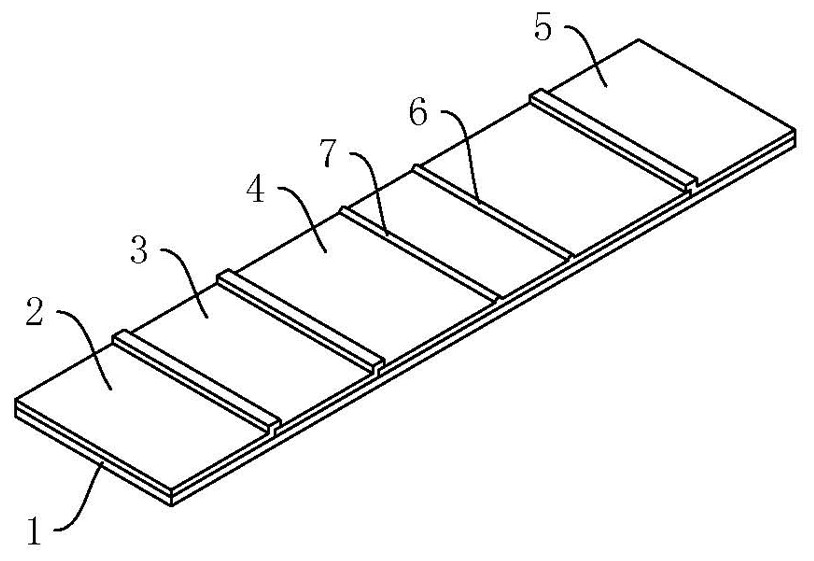
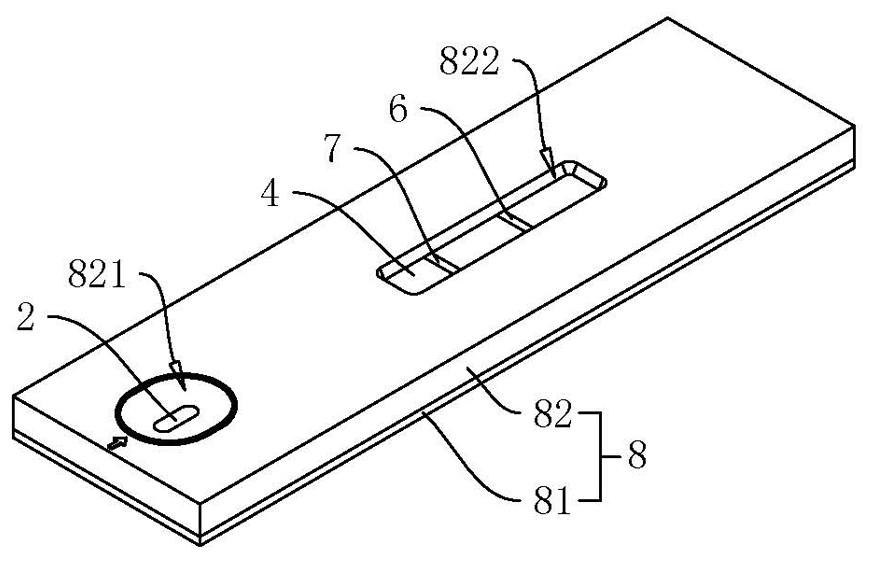
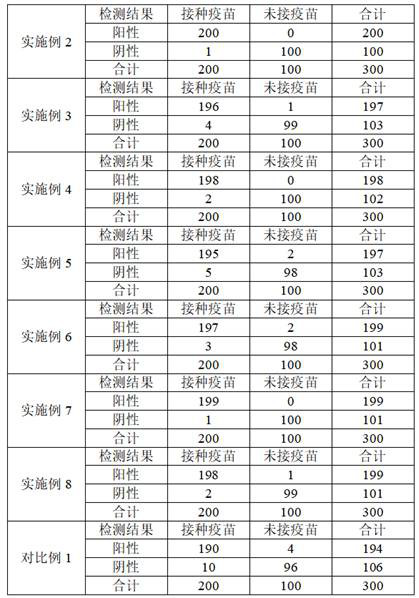
SARS-CoV-2 neutralizing antibody test strip and preparation method and kit thereof
A sars-cov-2 and test strip technology, applied in the field of SARS-CoV-2 neutralizing antibody test strips and its preparation, can solve the problems of inconvenient on-site detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Examples of Preparation of Raw Materials / Intermediates
[0043] Chicken IgY was purchased from Shanghai Yanmu Industrial Co., Ltd. (Cat. No. FK-025).
[0044] Goat anti-chicken secondary antibody was purchased from Luoyang Baitaike Biotechnology Co., Ltd., mg / mL titer (ELISA) > 1:100,000, polyacrylamide gel electrophoresis purity > 96%; storage buffer 0.01mol / L phosphate buffer, containing 0.14mol / L sodium chloride, pH 7.4; P300 preservative was purchased from SIGMA, Sinopharm USA.
[0045] Human IgG was purchased from Beijing Yita Biotechnology Co., Ltd., the product number is YT7150.
[0046] Anti-human IgG antibody was purchased from Shanghai Jianglai Biotechnology Co., Ltd., the product number is AbM59504-10-PU.
[0047] The SARS-CoV-2 S-RBD antigen was purchased from Nanjing Shenji Biotechnology Co., Ltd. The protein was constructed as a DNA sequence encoding the RBD region (Gln321-Ser591) of the spike protein of SARS-CoV-2 (2019-nCoV), and the expression host w...
preparation example 1
[0050] Preparation of colloidal gold
[0051] The preparation method of colloidal gold in the present application adopts sodium citrate reduction method to prepare colloidal nanoparticles, comprising the following steps:
[0052] (1) Take 99mL ultrapure water and 1mL 1wt% trichloroauric acid aqueous solution, mix well, stir with magnetic force and boil;
[0053] (2) Quickly add 1.8mL 1wt% trisodium citrate solution, continue heating and boiling;
[0054] (3) When the color of the colloidal gold solution changes from light yellow to blue-black, and finally to wine red, continue heating and boiling for 10 minutes;
[0055] (4) When the temperature of the prepared colloidal gold solution drops to room temperature, dilute the prepared colloidal gold solution to 100mL.
[0056] (5) Finally, put the prepared colloidal gold solution in a clean glass bottle and store it at 4°C for later use.
preparation example 2
[0058] Preparation of colloidal gold-labeled SARS-CoV-2 RBD antigen
[0059] The preparation method of the colloidal gold-labeled SARS-CoV-2 RBD antigen in the application comprises the following steps:
[0060] (1) Take 2mL of the colloidal gold solution prepared in Preparation Example 1, and use 0.1M K 2 CO 3 Adjust the pH to 8.0;
[0061] (2) Add 200 μL of SARS-CoV-2 S-RBD antigen with a concentration of 0.1ug / μL dropwise, and react with magnetic stirring for 2 hours at room temperature;
[0062] (3) Add 10wt% BSA solution dropwise to make the final concentration 0.5wt%, and react with magnetic stirring for 30min at room temperature:
[0063] (4) Add 10wt% PEG20000 solution dropwise to make the final concentration 0.2wt%, and stir magnetically for 30min at room temperature;
[0064] (5) Centrifuge at 12000rpm for 30min, discard the supernatant, and leave the colloidal gold precipitate;
[0065] (6) Add 1wt% BSA solution to the precipitate to resuspend the colloidal gol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com