Gynecological leucorrhea stopping sustained release tablet and preparation method thereof
A slow-release tablet and band-stop technology, which is applied in the field of gynecological band-stop sustained-release tablet and its preparation, can solve the problems of multiple medication administration and short drug action time, and achieves enhanced compliance, improved therapeutic effect and finished product quality, and improved The effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
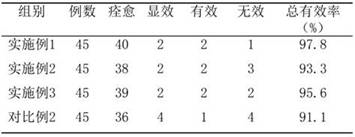
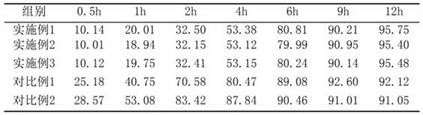
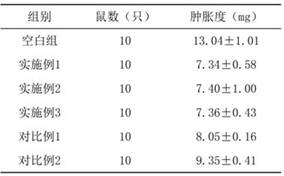
Embodiment 1
[0038] Embodiment 1, Fuke Zhitao Sustained Release Tablet of the present invention and its preparation method
[0039] Recipe: 363 g of toon bark, 64 g of schisandra, 363 g of Phellodendron cortex, 242 g of tortoise shell, 363 g of Poria cocos, 120 g of donkey-hide gelatin and 363 g of yam.
[0040] Main ingredient: 25 g concentrated dried Chinese medicine
[0041] Pharmaceutical excipients are: sodium polypropylene glycol diacetate 5g
[0042] Carmellose Sodium 10g
[0044] Microcrystalline Cellulose 100g
[0045]Preparation method: S1, medicinal material pretreatment;
[0046] S11. Cleaning: remove the non-medicinal parts, spoiled mildew, insects and sediment in the Chinese medicinal materials, and put them in a clean container;
[0047] S12. Washing: cleaning the medicinal material, washing away the sediment and unclean substances attached to the surface of the medicinal material, and soaking;
Embodiment 2
[0064] Example 2, Fuke Zhitao Sustained Release Tablet of the present invention and its preparation method
[0065] Recipe: 363 g of toon bark, 64 g of schisandra, 363 g of Phellodendron cortex, 242 g of tortoise shell, 363 g of Poria cocos, 120 g of donkey-hide gelatin and 363 g of yam.
[0066] Main ingredient: 25g concentrated dry Chinese medicine
[0067] Pharmaceutical excipients are: polypropylene glycol sulfate 5g
[0068] Hydroxypropyl Methyl Cellulose 10g
[0070] Microcrystalline Cellulose 105g
[0071] The preparation method is the same as in Example 1.
Embodiment 3
[0072] Example 3, Fuke Zhitao Sustained-release Tablet of the present invention and its preparation method
[0073] Recipe: 363 g of toon bark, 64 g of schisandra, 363 g of Phellodendron cortex, 242 g of tortoise shell, 363 g of Poria cocos, 120 g of donkey-hide gelatin and 363 g of yam.
[0074] Main ingredient: 25g concentrated dry Chinese medicine
[0075] Pharmaceutical excipients are: 1, 12-bis-histidine dodecyl diamine salt 4g
[0076] Povidone 10g
[0078] Microcrystalline Cellulose 105g
[0079] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com