Method for removing chlorine radicals and sulfate radicals in zinc hypoxide raw material
A technology of secondary zinc oxide and sulfate, applied in chemical instruments and methods, zinc oxide/zinc hydroxide, calcium/strontium/barium sulfate, etc., can solve the rising price of low-chlorine and low-sulfate raw materials and the reduction of electrowinning equipment Electric efficiency, pipeline blockage equipment efficiency, etc., to improve the added value of products, solve the enrichment of chloride and sulfate, and have the effect of strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
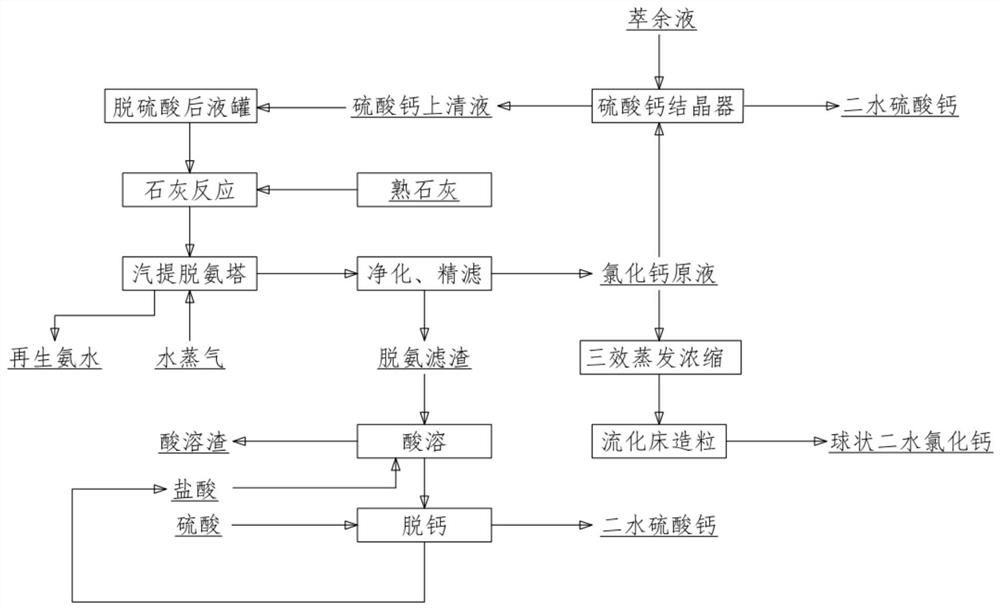
[0037] Will contain Cu: 0.05g / L, Cd: 0.05g / L, Pb: 1.5g / L, Zn: 1.0g / L, SO 4 2- : 48g / L, Cl - : 200g / L, NH 4+ : 1L of 100g / L raffinate and 600mL of 1mol / L calcium chloride solution were mixed by pumping at the same time. The feeding time was 1 hour. After reacting for 1.0h at 50°C, stop stirring and age for 1.0h. Then the mixed solution is suction-filtered with a vacuum pump and a Buchner funnel to obtain calcium sulfate solid and calcium sulfate supernatant, and the obtained calcium sulfate solid is dried at 60° C. for 24 hours to obtain a dry calcium sulfate dihydrate by-product.
[0038] Detection of NH in solution 4+ The content is 98.6g / L, and 184.1g of lime is put into the supernatant of calcium sulfate through the lime feeding system for pre-deamination. Above 12.0, the temperature of ammonium distillation is 80°C, and the time of ammonium distillation is 180min. The obtained ammonia gas is collected through an ammonia gas absorption tower to obtain regenerated ammoni...
Embodiment 2
[0042] Will contain Cu: 0.10g / L, Cd: 0.02g / L, Pb: 1.6g / L, Zn: 1.2g / L, SO 4 2- : 60g / L, Cl - : 196g / L, NH 4+ : 1L of 108g / L raffinate and 750mL of 1mol / L calcium chloride solution were mixed by pumping at the same time. The feeding time was 1 hour. After reacting for 1.0h at 60°C, stop stirring and age for 1.0h. Then the mixed solution is suction-filtered with a vacuum pump and a Buchner funnel to obtain calcium sulfate solid and calcium sulfate supernatant, and the obtained calcium sulfate solid is dried at 60° C. for 24 hours to obtain a dry calcium sulfate dihydrate by-product.
[0043] Detection of NH in solution 4+The content is 105.6g / L, and 197.1g of lime is put into the supernatant of calcium sulfate through the lime feeding system for pre-deamination. Above 12.0, the temperature of ammonium distillation is 85°C, and the time of ammonium distillation is 120min. The obtained ammonia gas is collected through an ammonia gas absorption tower to obtain regenerated ammoni...
Embodiment 3
[0047] Will contain Cu: 0.08g / L, Cd: 0.04g / L, Pb: 1.1g / L, Zn: 1.7g / L, SO 4 2- : 30g / L, Cl - : 200g / L, NH 4+ : 1L of 110g / L raffinate and 375mL of 1mol / L calcium chloride solution were mixed by pumping at the same time. The feeding time was 1 hour. After reacting for 1.0h at 60°C, stop stirring and age for 1.0h. Then the mixed solution is suction-filtered with a vacuum pump and a Buchner funnel to obtain calcium sulfate solid and calcium sulfate supernatant, and the obtained calcium sulfate solid is dried at 60° C. for 24 hours to obtain a dry calcium sulfate dihydrate by-product.
[0048] Detection of NH in solution 4+ The content is 106.3g / L, and 198.4g of lime is put into the supernatant of calcium sulfate through the lime feeding system for pre-deamination. Above 12.0, the temperature of ammonium distillation is 85°C, and the time of ammonium distillation is 180min. The obtained ammonia gas is collected through an ammonia gas absorption tower to obtain regenerated ammon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com