Flavane derivatives in dragon's blood and application of pharmaceutical composition of flavane derivative
A technology of drugs and compounds, applied in the field of medicine, can solve the problems of poor curative effect, high bleeding risk and unsatisfactory curative effect of ADP receptor inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
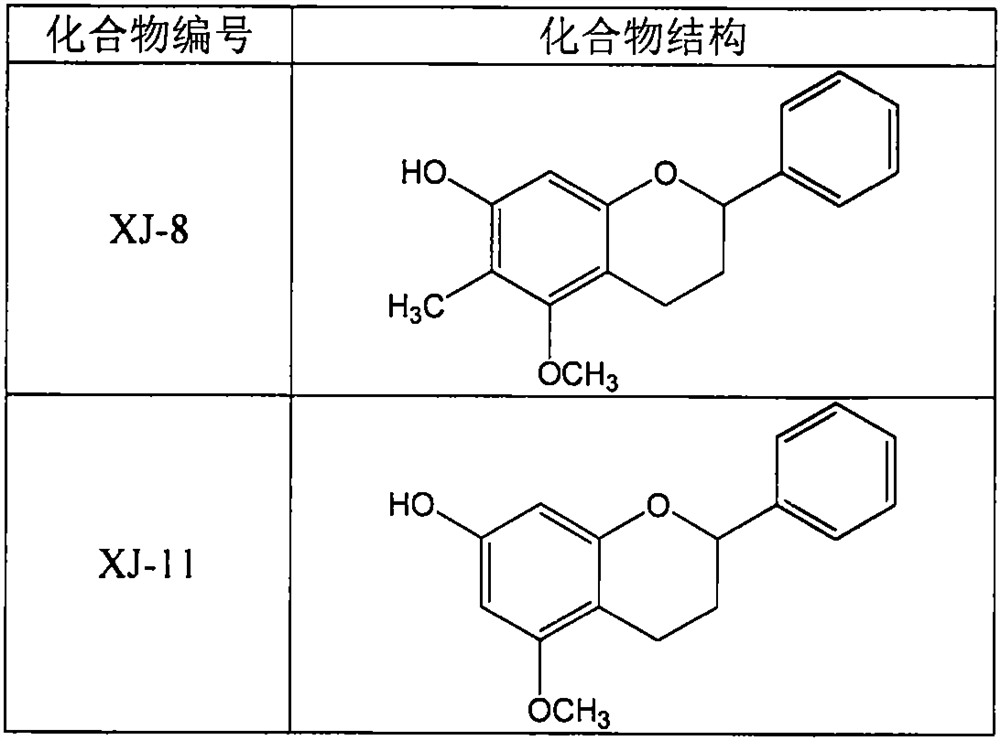
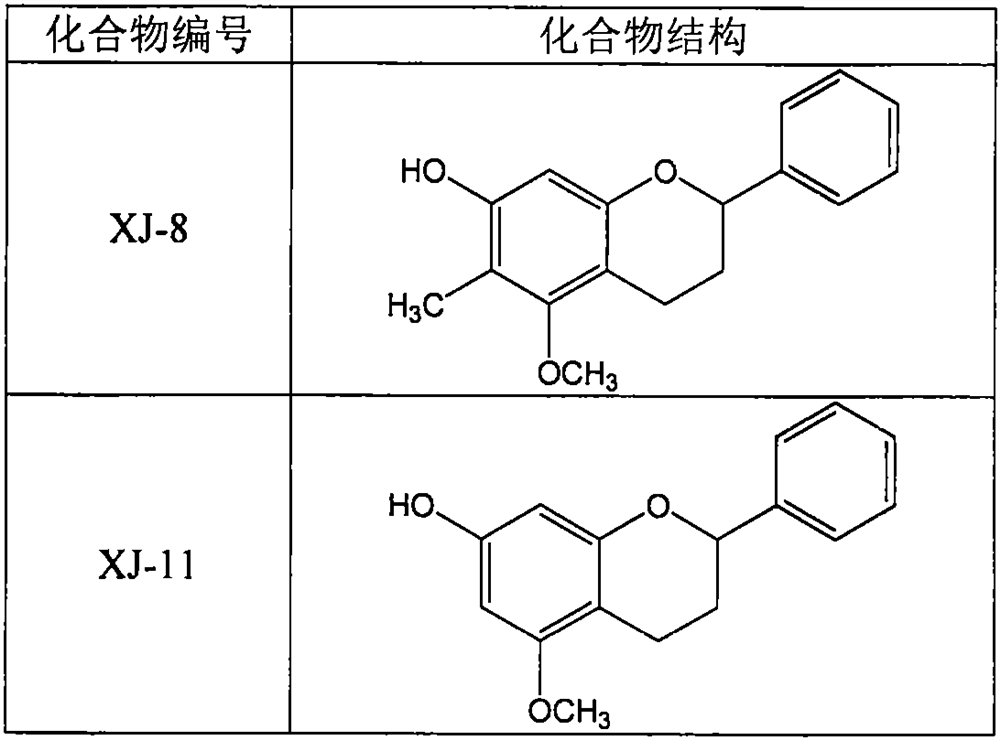
[0029] Example 1: Determination of the regulatory effect of compounds XJ-8 and XJ-11 on the release of ATP from rat platelets
[0030] Sprague Dawley rats were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg / kg), and blood was collected from the abdominal aorta by laparotomy into vacuum anticoagulated blood collection tubes containing sodium citrate. Centrifuge at 200g at room temperature for 10 minutes, take the upper platelet-rich plasma, then centrifuge at 1000g at room temperature for 10 minutes, and discard the supernatant. Add an equal volume of CGS buffer (containing 1.7mM citric acid, 18.3mM sodium citrate, 10mM glucose, 120mM sodium chloride, 2μN prostaglandin E1) to resuspend the platelets, centrifuge at 1000g for 10 minutes at room temperature, discard the supernatant, and repeat. Using modified bench solution (containing 137mM sodium chloride, 2.9mM potassium chloride, 0.34mM disodium hydrogen phosphate, 12mM sodium bicarbonate, 5mM 4-hydr...
Embodiment 2
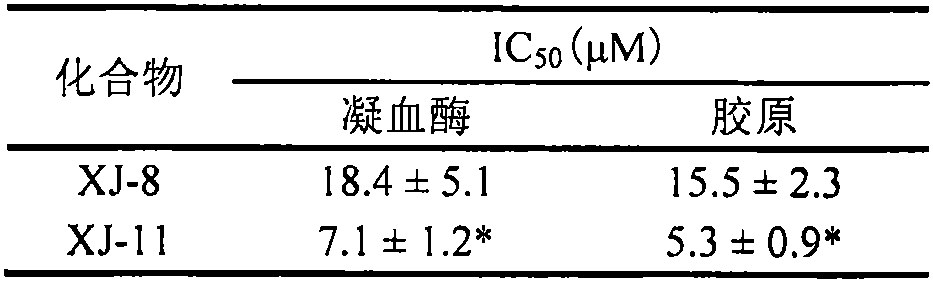
[0034] Example 2: Determination of the inhibitory effect of compounds XJ-8 and XJ-11 on rat platelet aggregation
[0035]Sprague Dawley rats were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg / kg), and blood was collected from the abdominal aorta by laparotomy into vacuum anticoagulated blood collection tubes containing sodium citrate. Centrifuge at 200 g for 10 minutes at room temperature, and take the upper layer of platelet-rich plasma. Take 200 μl of plasma into a platelet aggregation assay tube, add 25 μl of XJ-8 or XJ-11 prepared by modified benchtop solution to a final concentration of 5, 10, and 20 μM, incubate at 37°C for 10 minutes, and then add 25 μl of type I collagen to a final concentration of 5 μg / ml, start the platelet aggregation assay, and the assay time is 5 minutes. In addition, part of the platelet-rich plasma was centrifuged at 1000 g at room temperature for 10 minutes, and the supernatant was discarded. Add an equal volume of...
Embodiment 3
[0039] Example 3: Determination of the Effects of XJ-8 and XJ-11 on the Bleeding Time of Mice
[0040] 64 male BALB / c mice aged 8-10 weeks were randomly divided into 8 groups, control group (Control), positive drug aspirin (Aspirin) 200mg / kg group, XJ-8 and XJ-11 20, 40, 80mg / kg dose group. After the mice were fed adaptively for 3 days, aspirin, XJ-8 and XJ-11 were prepared as suspensions with 0.5% sodium carboxymethylcellulose aqueous solution, and then the mice in each administration group were given the corresponding doses of aspirin, XJ-11 by intragastric administration. 8 or XJ-11, the control group was given an equal volume of 0.5% sodium carboxymethylcellulose aqueous solution. After 3 hours of administration, 0.5 cm of the mouse tail was excised with a scalpel blade, and then the mouse tail was placed in a 5 ml centrifuge tube filled with 4 ml of 37°C normal saline, and the bleeding time was observed and recorded.
[0041] The results showed that the bleeding time (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com