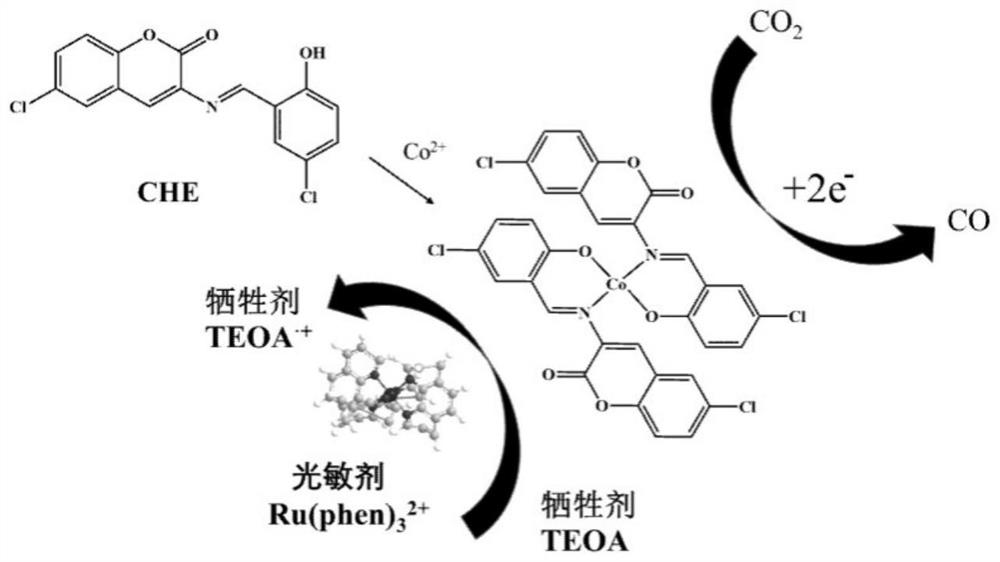
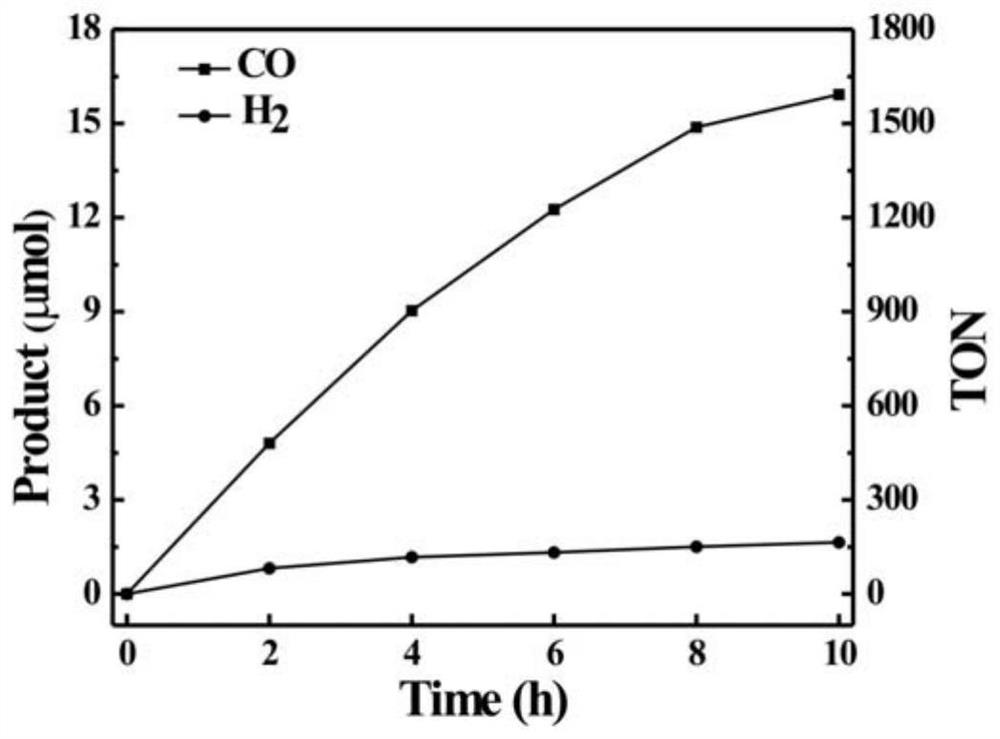
Preparation method and application of cobalt (II) coumarin Schiff base complex
A technology of coumarins and Schiff bases, which is applied in the direction of cobalt organic compounds, organic compounds/hydrides/coordination complex catalysts, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can Solve the problems of low reduction efficiency, low overpotential, and high reduction cost, and achieve the effects of less raw material consumption, high catalytic performance, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] In a 100mL round bottom flask, add 3.12g (20mmol) 5-chloro salicylaldehyde, 2.34g (20mmol) acetylglycine and 4.92g (60mmol) anhydrous sodium acetate, then add 40mL acetic anhydride, stir until the solid is completely dissolved , heated to reflux at 130°C for 12h, cooled to room temperature, added a large amount of ice water, and a large amount of precipitate was observed. After drying by suction, the product was placed in a mixed solvent of 20mL concentrated hydrochloric acid:ethanol (2:1) at 80 Reflux at ℃ for 5 hours, cool naturally, and adjust the pH to nearly neutral with 30% NaOH, a large amount of precipitates are precipitated, filtered and dried, and then recrystallized with absolute ethanol to obtain the precursor of 3-amino-6-chlorocoumarin;
[0048] Weigh 1.96g of 3-amino-6-chlorocoumarin (10mmol) and 1.56g of 5-chlorosalicylaldehyde (10mmol) and dissolve them in 20mL of absolute ethanol, reflux at 70°C for 6h, a large amount of precipitates are formed, cool A...
Embodiment 2
[0051] Add 3.12g (20mmol) 5-chloro salicylaldehyde, 2.34g (20mmol) acetylglycine and 4.92g (60mmol) anhydrous sodium acetate in a 100mL round bottom flask, then add 45mL acetic anhydride, stir until the solid is completely dissolved , heated to reflux at 135°C for 10 h, cooled to room temperature, added a large amount of ice water, and a large amount of precipitate was observed. After drying by suction, the product was placed in a mixed solvent of 20 mL concentrated hydrochloric acid:ethanol (2:1) at 80 Reflux for 4 hours at ℃, cool naturally, and adjust the pH to near neutral with 30% NaOH, a large amount of precipitate precipitates, filter and dry, and then recrystallize with absolute ethanol to obtain the precursor of 3-amino-6-chlorocoumarin;
[0052] Weigh 1.96g of 3-amino-6-chlorocoumarin (10mmol) and 1.56g of 5-chlorosalicylaldehyde (10mmol) and dissolve them in 20mL of absolute ethanol, reflux at 75°C for 5h, a large amount of precipitates are formed, cool After reachi...
Embodiment 3
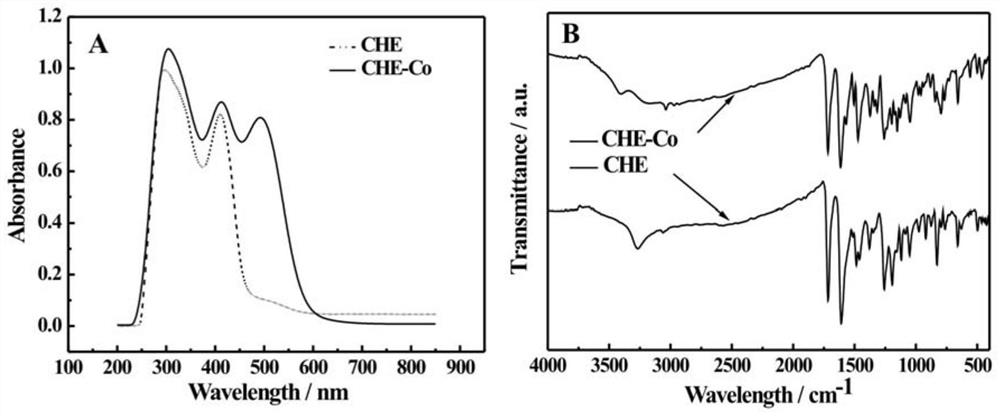
[0055] In a 100mL round bottom flask, add 3.12g (20mmol) 5-chloro salicylaldehyde, 2.34g (20mmol) acetylglycine and 4.92g (60mmol) anhydrous sodium acetate, then add 50mL acetic anhydride, stir until the solid is completely dissolved , heated to reflux at 140°C for 9h, cooled to room temperature, added a large amount of ice water, and a large amount of precipitate was observed. Reflux for 3 hours at ℃, cool naturally, and adjust the pH to nearly neutral with 30% NaOH, a large amount of precipitates are precipitated, filtered and dried, and recrystallized with absolute ethanol to obtain the precursor of 3-amino-6-chlorocoumarin; the Shimadzu The UV-2700 UV-visible spectrophotometer produced by the company records the UV-visible light absorption spectrum;
[0056] Weigh 1.96g of 3-amino-6-chlorocoumarin (10mmol) and 1.56g of 5-chlorosalicylaldehyde (10mmol) and dissolve them in 20mL of absolute ethanol, reflux at 80°C for 5h, a large amount of precipitates are formed, cool Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com