Method for selective hydrogenation of phenol on Na-modified NiCo catalyst
A technology for selective hydrogenation and catalyst, applied in the field of catalysis, which can solve the problems of low conversion rate and long reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This Na-modified NiCo catalyst was prepared by the following method:
[0037] 1) Preparation of catalyst precursor Na-NiCo@C:
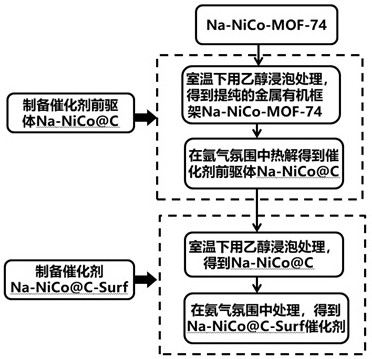
[0038] The synthetic route of Na-NiCo@C-Surf catalyst is as follows figure 1 As shown, the catalyst precursor Na-NiCo@C was first synthesized as follows:
[0039] Soak Na-NiCo-MOF-74 in ethanol solution at room temperature for 8 hours to remove surface residues, filter with suction, wash alternately with ethanol and water for 6 times, and dry in vacuum at 60°C for 18 hours to obtain pure Na-NiCo-MOF -74. Put pure Na-NiCo-MOF-74 in a tube furnace and pyrolyze it in an argon atmosphere. The temperature is raised from room temperature to 500 °C at a rate of 3 °C / min, kept at this temperature for 2 hours, and then dropped to room temperature , to obtain the carbon-coated nanoscale metal catalyst precursor Na-NiCo@C.
[0040] Wherein the preparation of Na-NiCo-MOF-74:
[0041] Add nickel acetate and cobalt acetate to distilled water to prepare ...
Embodiment 2
[0049] This Na-modified NiCo catalyst was prepared by the following method:
[0050] 1) Preparation of catalyst precursor Na-NiCo@C:
[0051] Soak Na-NiCo-MOF-74 in ethanol solution at room temperature for 24 hours to remove surface residues, filter with suction, wash with ethanol and water alternately 4 times, and dry in vacuum at 120°C for 8 hours to obtain pure Na-NiCo-MOF -74. Put pure Na-NiCo-MOF-74 in a tube furnace and pyrolyze it in an argon atmosphere. The temperature is raised from room temperature to 400 °C at a rate of 3 °C / min, kept at this temperature for 3 hours, and then dropped to room temperature , to obtain the carbon-coated nanoscale metal catalyst precursor Na-NiCo@C.
[0052] Wherein the preparation of Na-NiCo-MOF-74:
[0053] Add nickel acetate and cobalt acetate to distilled water to prepare a solution with a total metal salt concentration of 0.10 mol / L, wherein the molar ratio of nickel acetate and cobalt acetate is 1:4; add 2,5-dihydroxyterephthali...
Embodiment 3
[0059] This Na-modified NiCo catalyst was prepared by the following method:
[0060] 1) Preparation of catalyst precursor Na-NiCo@C:
[0061]At room temperature, the molar ratio of nickel and cobalt is 1:1.5, and the ratio of the molar weight of 2-hydroxypropane-1,2,3-tricarboxylic acid sodium to the total molar weight of the two metal salts is 1.5:7. -MOF-74 was soaked in ethanol solution for 16 hours to remove surface residues, filtered with suction, washed alternately with ethanol and water for 3 times, and dried in vacuum at 120°C for 8 hours to obtain pure Na-NiCo-MOF-74. Pure Na-NiCo-MOF-74 was placed in a tube furnace and pyrolyzed in an argon atmosphere. The temperature was raised from room temperature to 450 °C at a rate of 3 °C / min, kept at this temperature for 3 hours, and then dropped to room temperature , to obtain the carbon-coated nanoscale metal catalyst precursor Na-NiCo@C.
[0062] 2) Preparation of Na-NiCo@C-Surf catalyst:
[0063] Soak the obtained Na-Ni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com