Salt nanoparticles and compositions and methods of use thereof
A nanoparticle and composition technology, applied in nanomedicine, drug combination, nanotechnology, etc., can solve the problem that the release curve of stable encapsulating agent is not easy to control, and the therapeutic cytokine is easy to encapsulate and control release or combined delivery. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
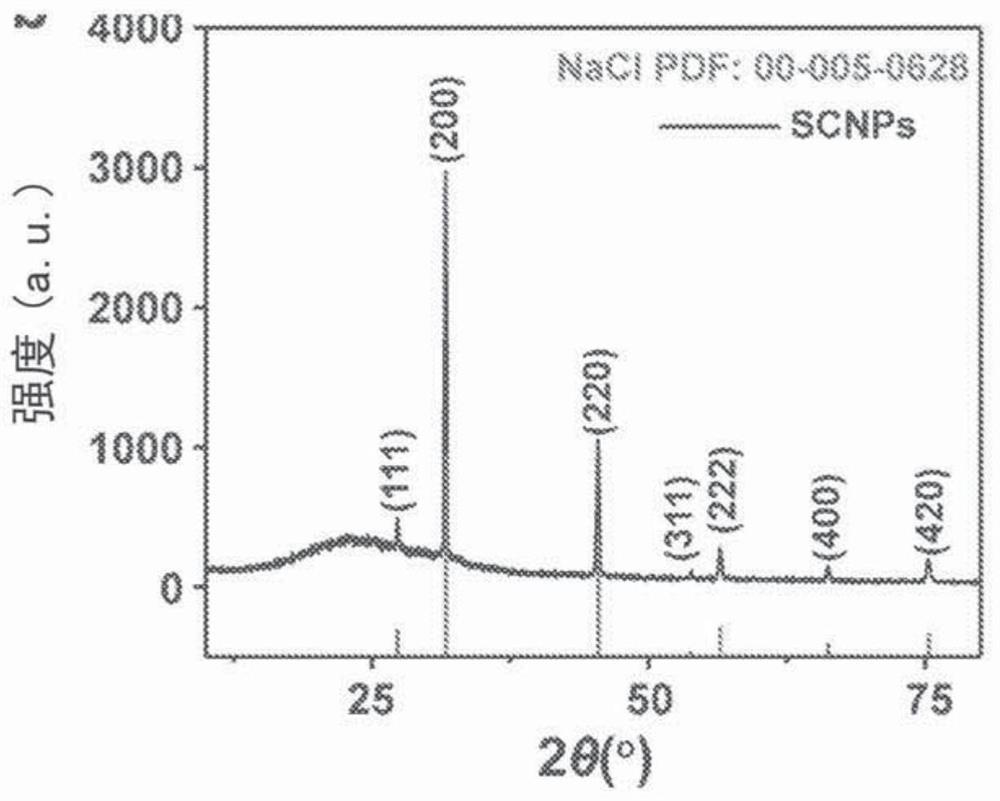
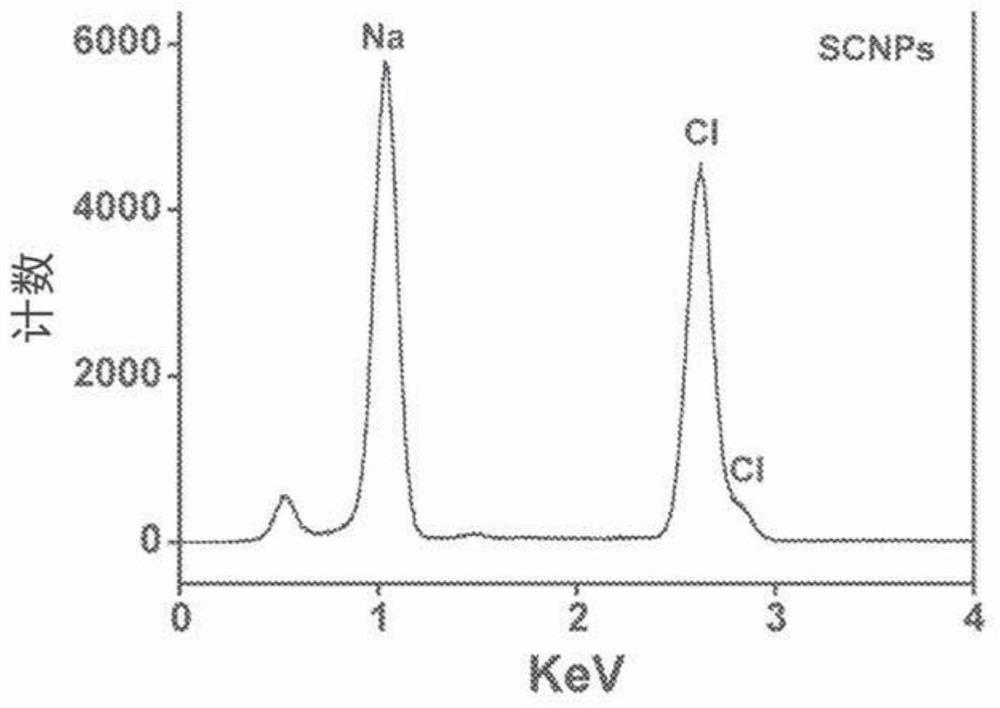
[0307] Example 1: Synthesis and degradation of NaCl nanoparticles.
[0308] Materials and methods
[0309] Synthesis of Sodium Chloride Nanoparticles (SCNP)
[0310] In a typical synthesis, 20 mg sodium oleate (TCI, 97%, lot number: W76EGFQ), 1 mL oleylamine (70%, Sigma-Aldrich, lot number: STBF9554V) and 50 mg 1,2-tetradecanediol (90% , Sigma-Aldrich) was dissolved in a mixed solution containing 10 mL of hexane (99.9%, Fisher) and 10 mL of ethanol (99.9%, Fisher). 15 mg of molybdenum(V) chloride (95%, Sigma-Aldrich, lot number: MKBQ9967V) was added to the mixture, and the solution was magnetically stirred at 60° C. for 24 hours. The crude product was collected by centrifugation at 12000 RPM for 10 minutes. The particles were redispersed in hexane by brief sonication and the centrifugation / hexane wash process was repeated 3 times to remove unreacted precursors.
[0311] Phospholipid-Coated Sodium Chloride Nanoparticles (PSCNP)
[0312] The above-synthesized SCNPs in hex...
Embodiment 2
[0323] Example 2: NaCl nanoparticles are taken up by cells and can be cytotoxic.
[0324] Materials and methods
[0325] cell culture
[0326] 4T1 (mouse breast carcinoma), HT29 (human colorectal adenocarcinoma), A549 (human lung adenocarcinoma), SGC7901 (human gastric adenocarcinoma), PC-3 (human prostate adenocarcinoma), UPPL-1541 (rat bladder carcinoma), t24, UMUC2 cells were grown in RPMI-1640 (Corning, 10-040-CV). U87MG (human glioblastoma) and RAW264.7 cells (murine macrophages) were grown in DMEM (Corning, 10-013-CV). B16-F10 (murine melanoma) and BBN963 cells in high glucose DMEM ( 30-2002 TM ) grows in. SCC VII cells (rat squamous cell carcinoma of the head and neck) in Grow in DMEM (Dulbecco's Modified Eagle Medium) / Hams F-12 50 / 50Mix (Corning, 10-090-CV). All cell culture media were supplemented with 10% fetal bovine serum (FBS) and 100 units / mL penicillin and 100 units / mL streptomycin (MediaTech, USA). Human primary prostate epithelial cells (HPrECs, AT...
Embodiment 3
[0338] Example 3: NaCl nanoparticles induce apoptosis of cancer cells.
[0339] Materials and methods
[0340] Mitochondrial potential (Δψm).
[0341]The change of mitochondrial membrane potential was measured by JC-1 mitochondrial membrane potential detection kit (Biotium, catalog number: 30001). Prepare JC-1 working solution by adding 10 µL of concentrated dye to 1 mL of RPMI medium without FBS. PSCNP (52.5, 105 or 160 μg / mL), PBS and NaCl (160.0 μg / mL in PBS) were incubated with cells for 6 hours. Remove medium and replace with JC-1 working solution and incubate for another 15 min. Stained cells were analyzed on an Array Scan VTI reader by analyzing Ch2 (green, JC-1 monomeric dye) and Ch3 (red, JC-1 aggregated dye) signals. Red / green ratios were analyzed by HCS Studio 2.0 Target Activation BioApplication software (Thermo Scientific, MA).
[0342] Oxygen Consumption Rate (OCR).
[0343] PC-3 cells (20,000 cells / well) were seeded in Seahorse XFe 24 assay plates and cult...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com