Immunoaffinity column based on bispecific monoclonal antibody for aflatoxin B1 and ochratoxin A and application of immunoaffinity column
A monoclonal antibody, aflatoxin technology, applied in the field of immunoaffinity column, can solve the problems of complicated operation, time-consuming and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 Aflatoxin B 1 (AFB 1 ) and the preparation of ochratoxin A (OTA) hybridoma cells
[0046] 1. Experimental method
[0047] 1. Aflatoxin B 1 Preparation of Artificial Antigen and Ochratoxin A Artificial Antigen
[0048] AFB 1 artificial antigen (AFB 1 -KLH and AFB 1 -OVA) is prepared as follows: 10mg AFB 1 and 20mg of carboxymethylhydroxylamine hemihydrochloride (CMO) were dissolved in a mixed solution of methanol-pyridine-water (volume ratio V:V:V=4:1:1), and dried to obtain a brown oil. Add an equal amount of chloroform and primary water to wash, collect the organic phase, and obtain the hapten AFB 1 -O. Take 2.5mg AFB 1 -O was dissolved in 200 μL DMF, then added equimolar 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) , stirred overnight at room temperature to obtain an activated solution. Add the activation solution dropwise to 2 mL of 5 mg / mL keyhole limpet hemocyanin (KLH) or 2.5 mg / mL ovalbu...
Embodiment 2
[0055] Example 2 HGPRT gene-deficient AFB 1 Mutation and Identification of Mutants in Hybridoma Cell Lines
[0056] 1. Experimental method
[0057] 1. AFB 1 HGPRT gene mutation mutagenesis treatment of hybridoma cell line
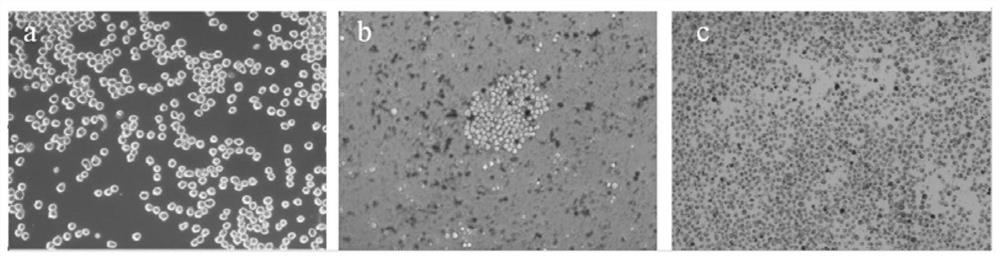
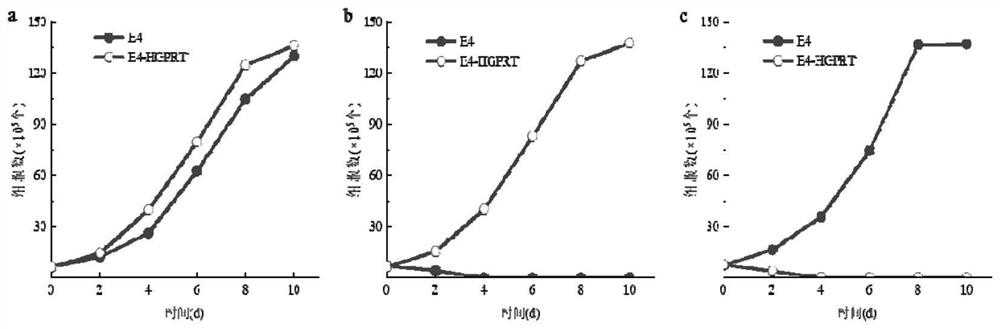
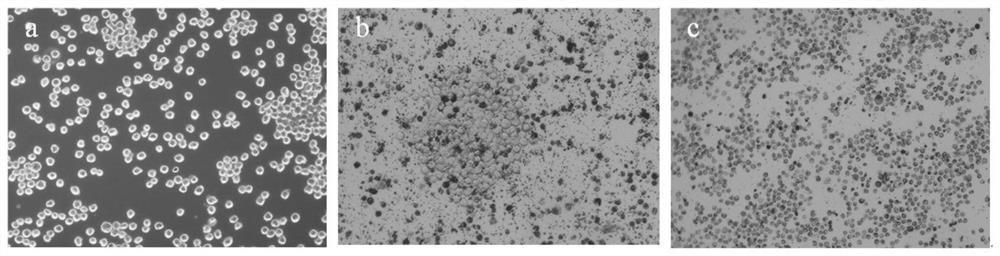
[0058] Before carrying out the induction treatment of cells, the AFB obtained in Example 1 1 The hybridoma cell line E4 was cultured in HAT medium for 2 days, and the culture condition was 5% CO 2 , 37°C; then cultured in HAT medium for 2 days to restore the cells to normal growth; take 5×10 5 AFB 1 The hybridoma cells were inoculated in a 10 cm culture dish containing HAT medium and cultured for 24 hours, then subjected to mutagenesis treatment, and added complete culture containing different concentrations of N-methyl-N'-nitro-nitrosoguanidine (MNNG) mutagen The concentration of MNNG in the culture medium was set to 5.0 μg / mL, 10.0 μg / mL, 20.0 μg / mL and 40.0 μg / mL respectively, and the culture time was set to 2 h, 4 h, 6 h and 10 h, respectively. A...
Embodiment 3
[0077] 1. Experimental method
[0078] The experimental method of this embodiment is basically the same as that of Example 2, the difference is that the cell lines that undergo HGPRT mutagenesis treatment are other AFBs obtained in Example 1 1 For the hybridoma cell lines E1-E3, the mutagenesis conditions were the optimal mutagenesis conditions determined in Example 2: the concentration of MNNG was 10 μg / mL, and the treatment time was 6 h.
[0079] 2. Experimental results
[0080] AFB 1 After the hybridoma cell lines E1-E3 were treated with the mutagenic conditions, the survival rates of the hybridoma cell lines were calculated by trypan blue staining, and the survival rates of E1-E3 were 68.45%, 70.20%, and 69.03%, respectively. It shows that the optimized mutagenesis conditions in Example 2 can obtain HGPRT-deficient cells with a relatively suitable and stable survival rate, which can be used in cell fusion experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com