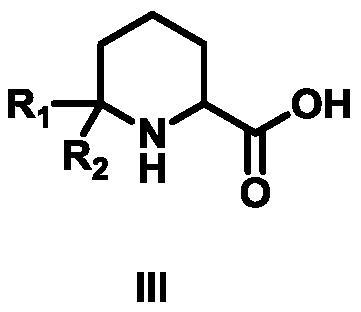
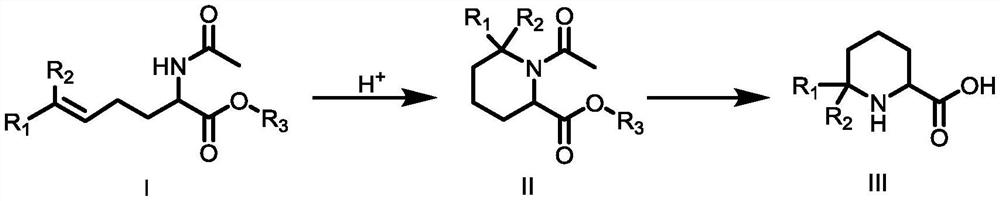
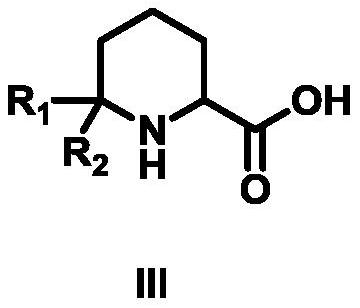
Synthesis method of 6,6-dialkyl piperidine-2-carboxylic acid compound
A synthetic method and compound technology, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The first step: the synthesis of the compound 1-acetyl-6,6-dimethylpiperidine-2-carboxylic acid ethyl ester
[0031] Add 2-(acetylamino)-6-methyl-5-heptanoic acid ethyl ester (10g, 44mmol, 1eq) into 100ml of trifluoroacetic acid, react at 50°C for 2 hours until detected by TLC Reach the end of the reaction, cool to room temperature, add to ice water, adjust the pH to 8-9 with sodium bicarbonate, extract twice with 600mL ethyl acetate, combine the organic phases and backwash with saturated brine, add anhydrous sodium sulfate to dry, Distilled under reduced pressure and eluted by column chromatography. The eluent used in column chromatography was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 5:1 to obtain 8.1 g of compound 1-acetyl-6,6- Ethyl dimethylpiperidine-2-carboxylate, the yield is 81.00%, the purity is 98%, 1 HNMR (500MHz, CDCl 3 )δ4.66(t, J=8.0Hz, 1H, NCH), 4.14(q, J=6.0Hz, 2H, CH 2 CH 3 ), 2.22 (ddd, J=18.2Hz, 7.9Hz, 5.6Hz, 1H, ...
Embodiment 2
[0038]Step (1) in embodiment 2 to embodiment 3 adopts the acid solution different from embodiment 1 to carry out reaction, and embodiment 2 adopts glacial acetic acid to carry out reaction, with respect to embodiment 1, yield decreases to some extent, is 70.00%. In particular, in Example 3, hydrochloric acid is used for the reaction, not only the reaction rate is obviously slow, but also more by-products are generated during the heating process, which makes the reaction yield significantly lower than that of Example 1, only 23.01%.
Embodiment 4
[0039] Step (1) in embodiment 4 to embodiment 5 adopts the reaction temperature different from embodiment 1. With respect to embodiment 1, embodiment 4 reduces reaction temperature to 25 ℃, and reaction rate obviously reduces, and the reaction yield in the same reaction time reduces to some extent, only 9.30%; embodiment 5 raises reaction temperature to 70 ℃, has promoted The occurrence of side reactions reduces the reaction yield to 75.00% compared to Example 1.
[0040] Step (2) in embodiment 6 to embodiment 7 adopts the sodium hydroxide of different consumption with embodiment 1 to react, with respect to embodiment 1, embodiment 6 and embodiment 7 reduce sodium hydroxide with respect to embodiment 1 respectively to 3 equivalents of ethyl 1-acetyl-6,6-dimethylpiperidine-2-carboxylate and to increase the amount of sodium hydroxide to 1-acetyl-6,6-dimethylpiperidine- 5 equivalents of ethyl 2-carboxylate, the reaction yield is slightly lower than that of Example 1, being 72.70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com