Preparation method of cathode oxygen reduction reaction catalyst based on two-dimensional graphite phase carbon nitride cobalt doped porous carbon material
A technology of cobalt carbonitride and porous carbon materials, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of toxicity, single synthesis cost of precursors, and high cost of platinum-based catalytic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
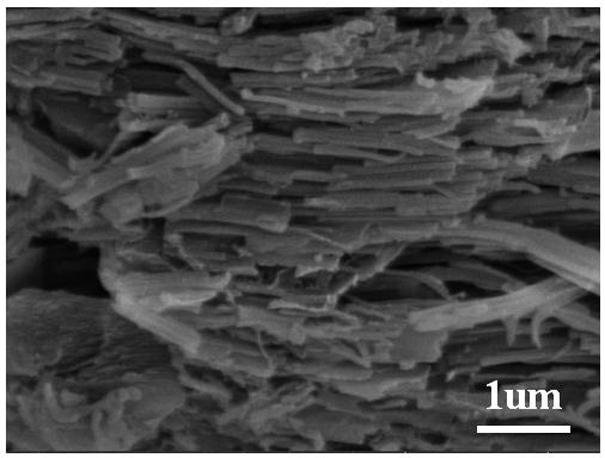
[0047] The invention provides a method for preparing a porous carbon material based on two-dimensional graphite phase carbon nitride cobalt doping and using the material as an oxygen reduction catalyst.
[0048] The active substance described in the present invention is abbreviated as C 3 N 4 @Co-BDC-TA.
[0049] C in the present invention 3 N 4 @Co-BDC-TA composites are prepared by thermal decomposition.
[0050] The present invention uses a platinum electrode as a counter electrode, and a saturated silver chloride electrode (Ag / AgCl) as a reference electrode respectively, C 3 N 4 A glassy carbon electrode made of @Co-BDC-TA composite was used as the working electrode.
[0051] A catalyst ink (ink) was prepared by weighing 4 mg of the catalyst of the present invention with a balance and dispersing it in 1 mL of a mixed solution (235 μL of deionized water, 750 μL of isopropanol and 15 μL of 5 wt% Nafion solution). Then gradually drop 28 μL of ink onto the surface of the...
Embodiment 1
[0060] This example shows a C 3 N 4 The synthetic method of @Co-BDC-TA composite material, comprises the following steps:
[0061] (1) Put a certain amount of melamine in a 50 mL ceramic crucible (with lid);
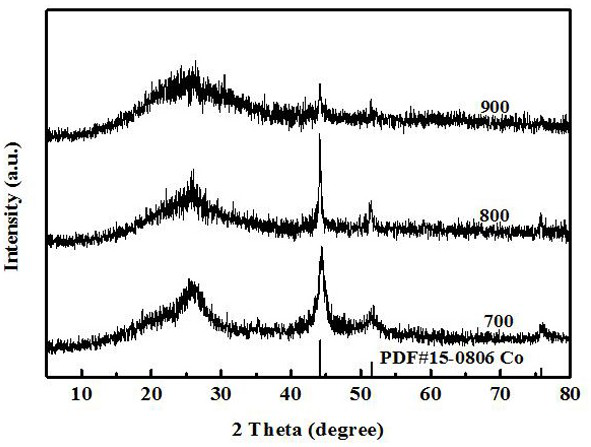
[0062] (2) Heat the crucible to 555 °C in a muffle furnace, keep it for 4 h, and cool down naturally to obtain a light yellow powder g-C 3 N 4 ;
[0063] (3) Weigh 400 mg of light yellow powder g-C obtained in step (2) 3 N 4 , dispersed in 40 mL of absolute ethanol and ultrasonicated for 30 min to obtain liquid A;
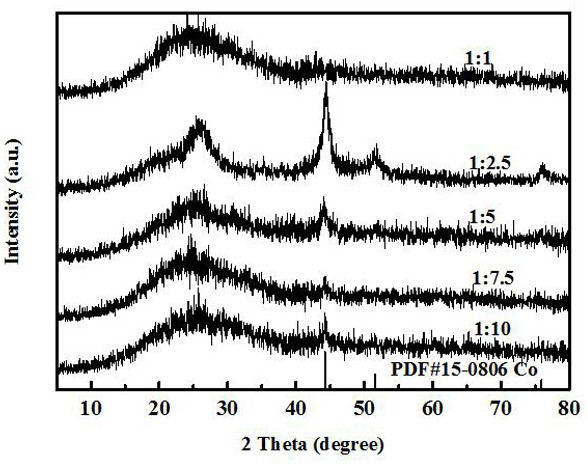
[0064] (4) Dissolve 84.7 mg of terephthalic acid (BDC) and 36.4 mg of cobalt nitrate hexahydrate (1:2.5) in 10 mL of TA solution (5 mmol / L, 0.8506 g of tannin in 100 mL of deionized water ), to obtain liquid B;
[0065] (5) Slowly add solution B into solution A which is magnetically stirred at 50 °C and 800 rpm, and keep stirring for 6 h;
[0066] (6) Evaporate the solution obtained in step (5) to dryness in a water bath at 80 °C, and dry it under va...
Embodiment 2
[0069] (1) Put a certain amount of melamine in a 50 mL ceramic crucible (with lid);
[0070] (2) Heat the crucible to 555 °C in a muffle furnace, keep it for 4 h, and cool down naturally to obtain a light yellow powder g-C 3 N 4 ;
[0071] (3) Weigh 400 mg of light yellow powder g-C obtained in step (2) 3 N 4 , dispersed in 40 mL of absolute ethanol and ultrasonicated for 30 min to obtain liquid A;
[0072] (4) Dissolve 84.7 mg terephthalic acid (BDC) and cobalt nitrate hexahydrate in 10 mL TA solution (5 mmol / L, 0.8506 g tannins were dissolved in 100 mL deionized water) to obtain liquid B;
[0073] (5) Slowly add solution B into solution A which is magnetically stirred at 50 °C and 800 rpm, and keep stirring for 6 h;
[0074] (6) Evaporate the solution obtained in step (5) to dryness in a water bath at 80 °C, and dry it under vacuum at 80 °C overnight to obtain C 3 N 4 @Co-BDC-TA powder;
[0075] (7) Put the vacuum-dried product in a tube furnace (argon atmosphere) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Limiting current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com