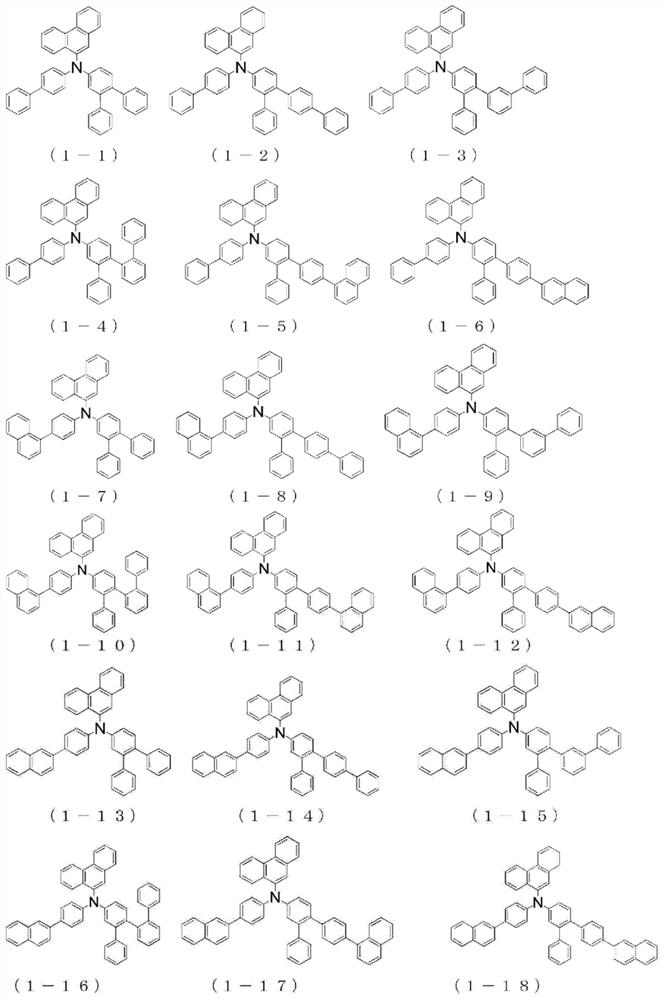
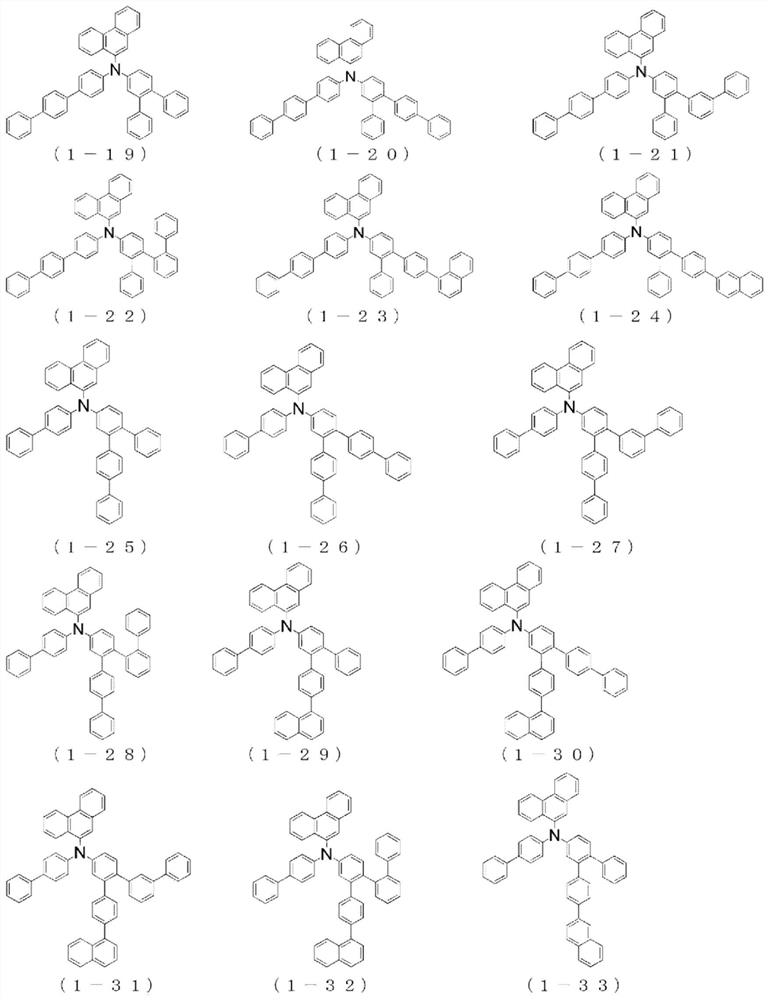
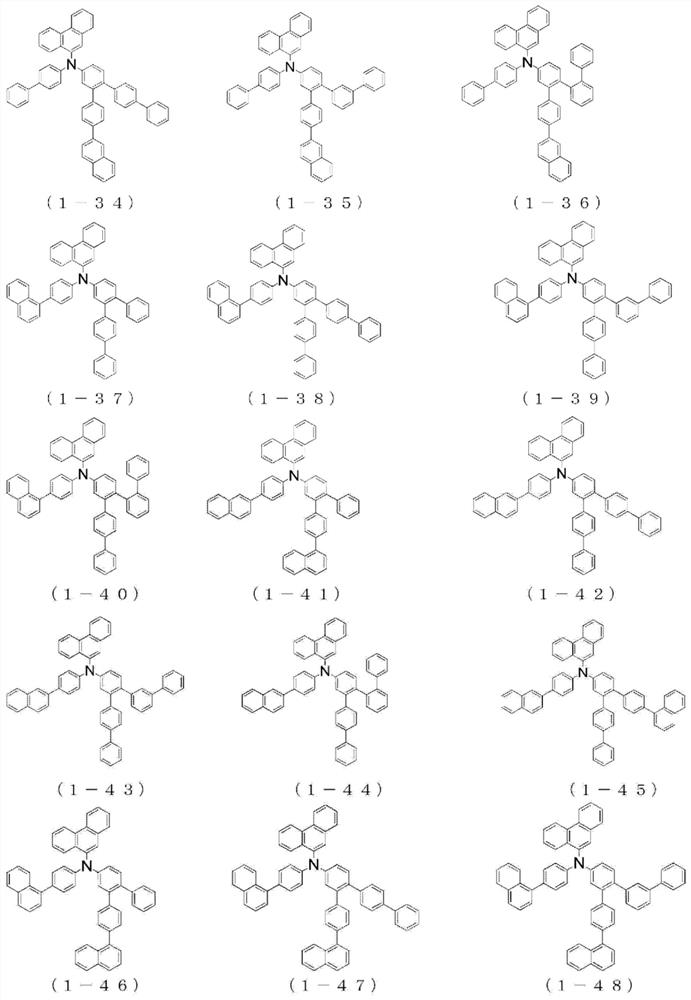Organic electroluminescent device
An electroluminescent element and electroluminescent technology, applied in electrical elements, luminescent materials, organic chemistry, etc., can solve problems such as insufficient, low driving voltage luminous efficiency, and reduced element characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227]
[0228] 22.4 g of 4-(naphthalen-1-yl)aniline, 19.8 g of 3-bromobiphenyl, 12.2 g of sodium tert-butoxide, and 230 mL of toluene were added to a nitrogen-substituted reaction vessel, and nitrogen gas was blown in while irradiating ultrasonic waves for 30 minutes. 0.8 g of tris(dibenzylideneacetone)dipalladium and 0.5 g of 2,2'-bis(diphenylphosphine)-1,1'-binaphthyl were added, heated, and stirred at 110°C for 16 hours. After removing insoluble matter by filtration, the filtrate was concentrated. The residue was purified by column chromatography to obtain 25.4 g (yield 75.6%) of 4-(naphthalen-1-yl)phenyl-biphenyl-3-amine as a light yellow viscous substance.
[0229] 23.9 g of 4-(naphthalen-1-yl)phenyl-biphenyl-3-amine and 240 mL of dimethylformamide were added to the nitrogen-substituted reaction vessel, and cooled in an ice bath. 11.6 g of N-bromosuccinimide was added in portions over 30 minutes, followed by stirring at 5° C. for 4 hours. Water was added, extracted w...
Embodiment 2
[0238]
[0239] Add (1,1':2',1"-terphenyl-4'-yl)amine 17.2g, 2-(4-bromophenyl)naphthalene 18.0g, tert-butoxy Sodium 9.2g, toluene 270mL, while irradiating ultrasonic waves for 30 minutes, pass nitrogen gas. Add tris(dibenzylideneacetone) dipalladium 1.2g, 2,2'-bis(diphenylphosphine)-1,1'-bis 0.8 g of naphthalene was heated and stirred at 110° C. for 16 hours. After removing the insolubles by filtration, the filtrate was heated, and at 80° C., adsorption purification using activated clay and silica gel was carried out, and heating and filtration was carried out. The filtrate was concentrated and passed through toluene-heptyl The residue was purified by recrystallization in alkanes to obtain {4-(naphthalene-2-yl)phenyl}-(1,1':2',1"-terphenyl-5'-yl)amine as a yellow-white powder Body 24.3g (yield 85.4%).
[0240] Add 24.3 g of {4-(naphthalene-2-yl)phenyl}-(1,1':2',1"-terphenyl-5'-yl)amine, 9-bromo 15.4g of phenanthrene, 10.4g of sodium tert-butoxide, 240mL of toluene, while i...
Embodiment 3
[0247]
[0248] The {4"-(naphthalene-1-yl)-1,1':2',1"-terphenyl-5'-yl}-{4-(naphthalene-1-yl)phenyl group of Example 1 } amine to {4"-(naphthalen-1-yl)-1,1':2',1"-terphenyl-5'-yl}-{4-(naphthalen-2-yl)phenyl} Amines, the same procedure was performed to give {4-(naphthalene-2-yl)phenyl}-(phenanthrene-9-yl)-{4"-(naphthalene-1-yl)-1,1':2', 4.9 g of white powder of 1"-terphenyl-5'-yl}amine (compound 1-17) (yield 40%).
[0249] The structure of the obtained white powder was identified using NMR.
[0250] use 1 H-NMR (CDCl 3 ) detected the following 39 hydrogen signals.
[0251] δ(ppm)=8.76-8.86(2H), 8.24-8.28(1H), 8.06(1H), 7.85-7.95(8H), 7.36-7.78(19H), 7.19-7.31(8H).
[0252] [chem 21]
[0253]
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com