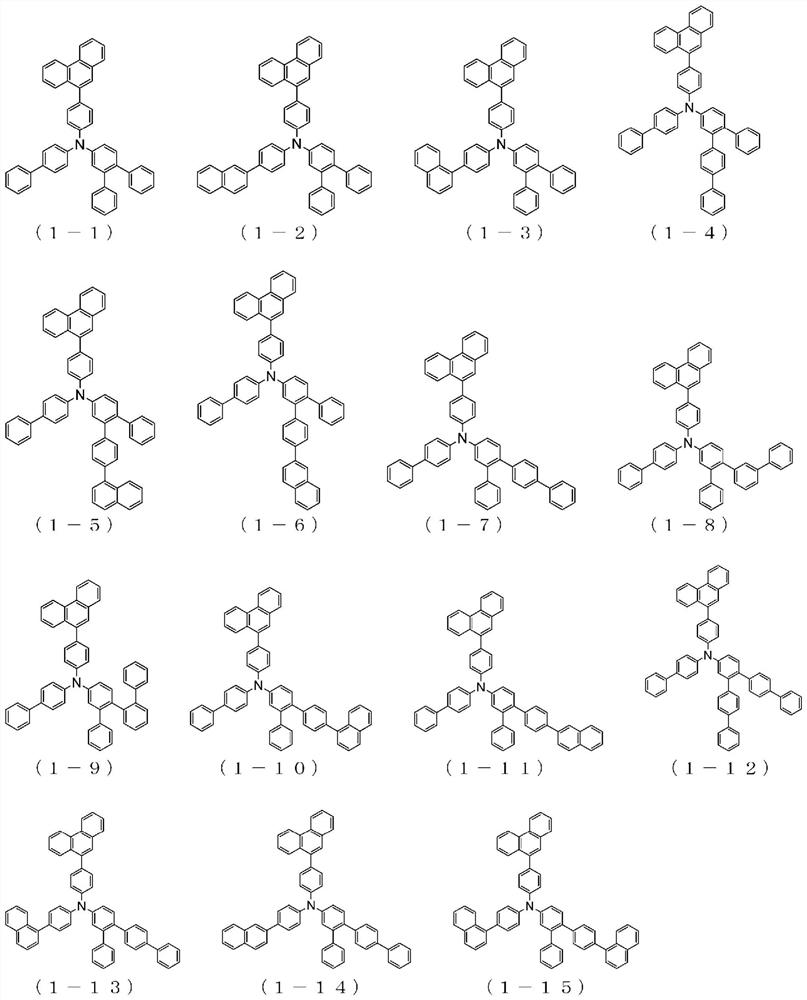
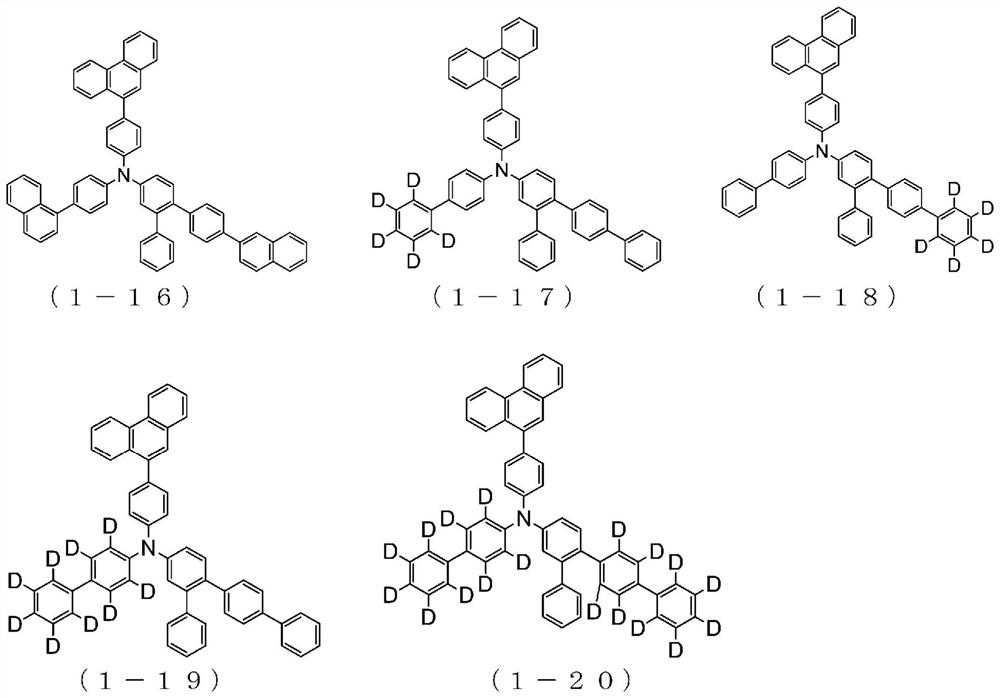
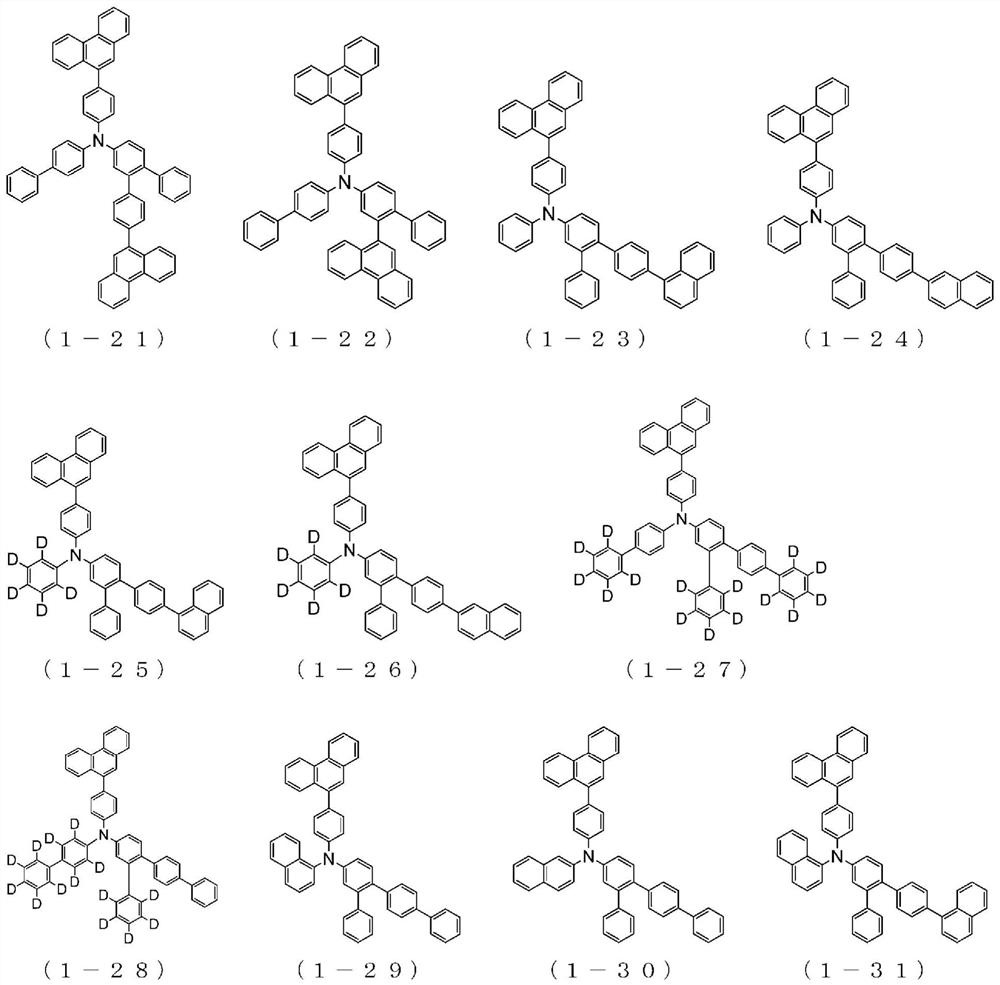
Organic electroluminescent element
An electroluminescent element and luminescent technology, applied in the direction of electrical components, organic chemistry, luminescent materials, etc., can solve problems such as unexpectable luminous efficiency, insufficient electron barrier property, and reduced component characteristics, and achieve excellent electron blocking ability , Excellent electron blocking ability, and the effect of low driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125]
[0126] In the reaction vessel was added (4-naphthalen-2-yl-phenyl)-[1,1';2',1"]terphenyl-4'-yl-amine: 15.0 g, 9-(4-bromo- Phenyl)-phenanthrene: 12.3g, sodium tert-butoxide: 4.8g, toluene: 240mL, pass nitrogen gas while irradiating ultrasonic waves for 30 minutes. Then, add palladium acetate (II): 0.1g, tri(tert-butyl) Phosphine: 0.3g, refluxed and stirred for 4 hours. After natural cooling, the filtrate obtained by filtration was concentrated under reduced pressure to obtain a crude product. The crude product obtained was purified by recrystallization utilizing a toluene solvent to obtain (4-naphthalene- White powder of 2-yl-phenyl)-(4-phenanthren-9-yl-phenyl)-[1,1';2',1"]terphenyl-4'-yl-amine (1-2) Body: 18.7 g (80% yield).
[0127] [hua 5]
[0128]
[0129] The structure of the obtained white powder was identified using NMR.
[0130] use 1 H-NMR (CDCl 3 ) detected the following 37 hydrogen signals.
[0131] δ(ppm)=8.81(1H), 8.76(1H), 8.11(1H), 8.09(1H), 7...
Embodiment 2
[0133]
[0134] In the reaction vessel was added (4-phenanthren-9-yl-phenyl)-[1,1';2',1"]terphenyl-4'-yl-amine: 15.0 g, 1-(4-bromo- Phenyl)-naphthalene: 9.4g, sodium tert-butoxide: 4.3g, toluene: 210mL, while irradiating ultrasonic wave for 30 minutes and passing nitrogen gas. Then, add palladium acetate (II): 0.1g, tri(tert-butyl) Phosphine: 0.1 g, refluxed and stirred overnight. After natural cooling, the filtrate obtained by filtration was concentrated under reduced pressure to obtain a crude product. The obtained crude product was subjected to column chromatography (carrier: silica gel, eluent: dichloromethane). / n-heptane) was purified to obtain (4-naphthalen-1-yl-phenyl)-(4-phenanthren-9-yl-phenyl)-[1,1';2',1"]triple White powder of phenyl-4'-yl-amine (1-3): 15.9 g (yield 76%).
[0135] [hua 6]
[0136]
[0137] The structure of the obtained white powder was identified using NMR.
[0138] use 1 H-NMR (CDCl 3 ) detected the following 37 hydrogen signals.
[013...
Embodiment 3
[0141]
[0142] Add biphenyl-4-yl-(6-bromo-[1,1';4',1"]terphenyl-3-yl)-(4-phenanthren-9-yl-phenyl)- Amine: 10.0 g, phenylboronic acid: 2.5 g, potassium carbonate: 3.6 g, toluene: 80 mL, ethanol: 30 mL, H 2O: 30 mL, and nitrogen gas was introduced while irradiating ultrasonic waves for 30 minutes. Then, tetrakis(triphenylphosphine)palladium(0):0.3 g was added, and the mixture was stirred under reflux overnight. After being left to cool, the organic layer was subjected to liquid separation extraction by a liquid separation operation, and concentrated under reduced pressure to obtain a crude product. The obtained crude product was purified by crystallization using a dichloromethane / acetone mixed solvent to obtain biphenyl-4-yl-(4-phenanthren-9-yl-phenyl)-[1,1';2', White powder of 1"; 4", 1"'] tetraphenyl-4'-yl-amine (1-4): 5.4 g (yield 54%).
[0143] [hua 7]
[0144]
[0145] The structure of the obtained white powder was identified using NMR.
[0146] use 1 H-NMR (CDC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com