Metal complex and electron transport material using same
A technology of electron transport materials and metal complexes, applied in the direction of 1/11 group organic compounds without C-metal bonds, materials of organic semiconductor devices, lithium organic compounds, etc. Research on multilayer structure durability and other issues, to achieve the effect of excellent luminous efficiency, excellent durability, and high electron injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
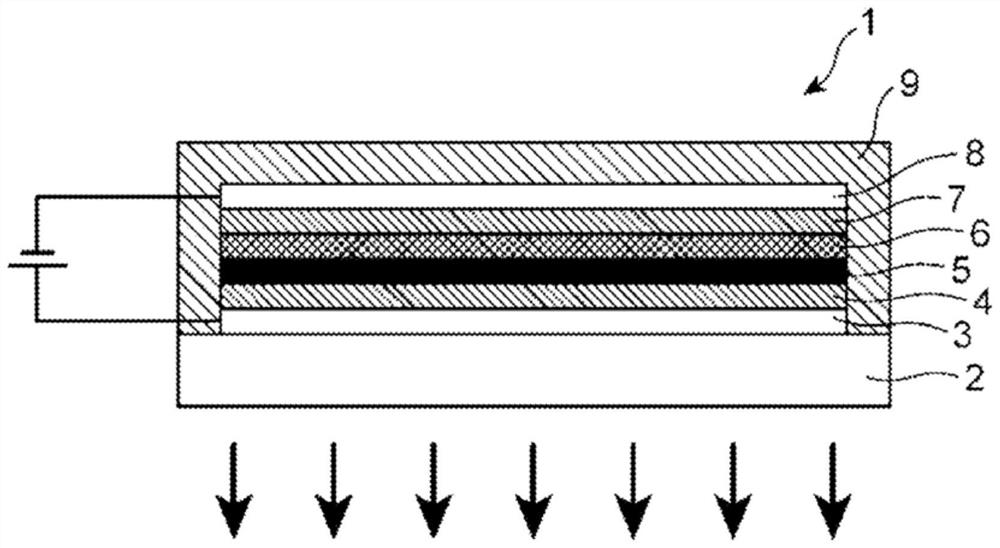
[0322] As the solvent or dispersion medium used in the preparation for the formation of the light-emitting layer, the same substances as those described in the formation of the hole injection layer 4 and the hole transport layer 5 can be used, but can be selected according to the hole transport layer 5 to be formed. The formed hole transport layer 5 is insoluble in the solvent.
[0323] Next, the electron-transporting layer 7 is formed on the light-emitting layer 6 according to the following steps, for example.
[0324] (a) The first process
[0325] First, a liquid material for forming an electron transport layer containing a metal complex represented by the above formulas (1) to (3) and, if necessary, a dopant such as a metal alkoxide is prepared.
[0326] As the solvent used for the preparation of the liquid material for forming the electron transport layer, a solvent that hardly swells or dissolves the constituent materials of the light emitting layer 6 is preferable, and...
Embodiment
[0342] Compound identification was performed by thin layer chromatography analysis, FAB, MS or ASAP-TOF-MS. FAB and MS were measured using JMS700 manufactured by JEOL Ltd. ASAP-TOF-MS used LCT Premier XE manufactured by Waters Corporation.
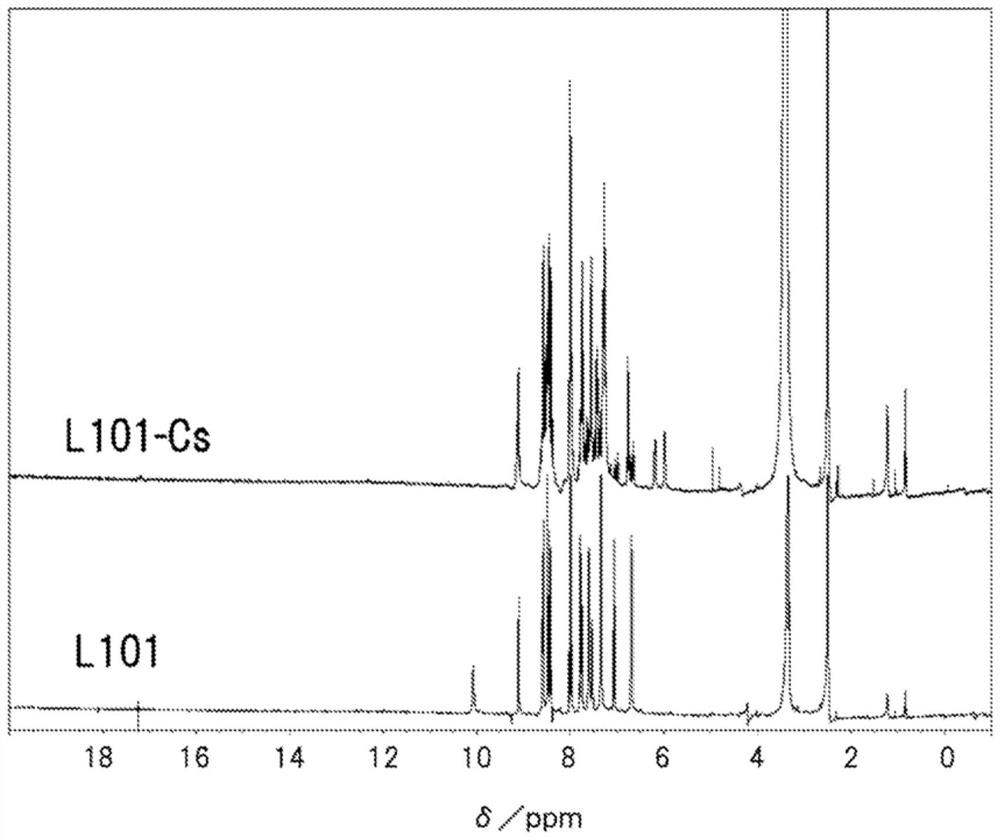
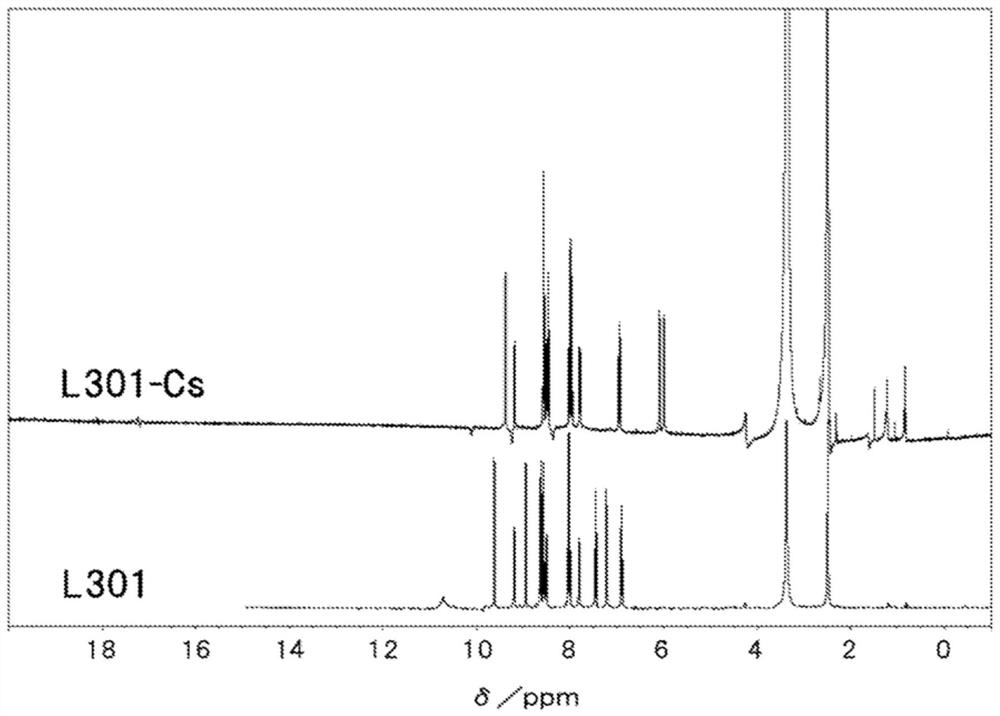
[0343] Also, for several ligands and complexes, DMSO-d6 was used as a deuterated solvent, and NMR (400 MHz) was measured with JNM-LA400 manufactured by JEOL Ltd.
[0344] In addition, as the silica gel C300 used for column chromatography, Wakosil C300 (C300) manufactured by Wako Pure Chemical Industries, Ltd., and Chromatorex NH manufactured by Fuji Silysia Chemical Co., Ltd. were used. 2 (NH 2 ).
[0345] [1] Synthesis of metal complexes
[0346] [A] Metal complex represented by formula (1)
[0347] [A-1] Synthesis of L101-M complex (M=Cs, Rb)
[0348] [A-1-1] Synthesis of Ligand L101
[0349] (1-1-1) Synthesis of intermediates: Synthesis of N-(3-chlorophenyl)-3-methoxy-2-nitrosoaniline
[0350] [chemical formula 39]
[0351] ...
Embodiment 3
[0602] The relative lifetime is based on the lifetime of Example 3 [material complex (L301-Cs)+dopant (LiOBu)+electroinjection layer] (100).
[0603] (4) Examples, Comparative Examples
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com