4-fluorine substituted arylamine compound and synthesis method thereof
A kind of technology of aniline compound and synthesis method, applied in the direction of amino compound preparation, amino hydroxyl compound preparation, chemical instrument and method, etc., can solve the problems of expensive reagents, unsuitable for scale-up production, etc., achieve low cost, widely popularize and use, increase lipophilic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
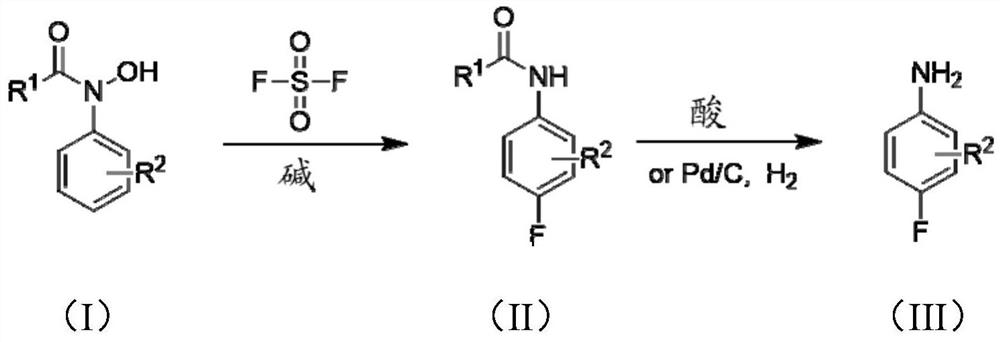
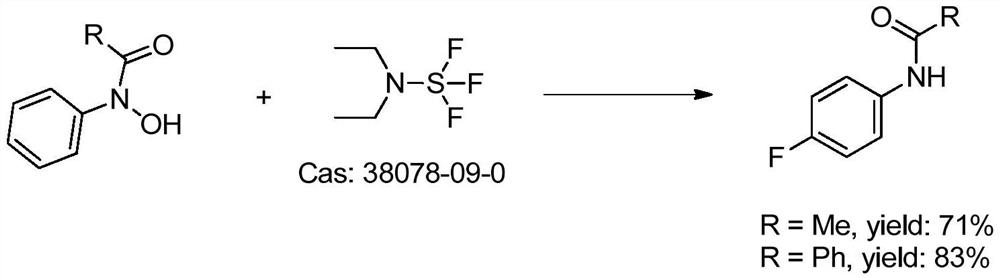
[0046] Add N-acetylphenylhydroxylamine (151g, 1.0mol), KF (29g, 0.5mol) and dichloromethane (1.0L) to a stirred 2L reaction flask in turn, cool to 0°C, and pass through sulfonyl fluoride (202g, 2mol), seal the system, slowly warm up to room temperature, stir and react for 12h, add saturated brine (500mL) to wash after the reaction, the organic phase is concentrated under reduced pressure to distill dichloromethane (recycling), and the crude product is purified under reduced pressure. Distillation to obtain white solid N-acetyl 4-fluoroaniline (142g), N-acetyl 4-fluoroaniline was added to 2mol / L hydrochloric acid (500mL), heated to reflux for 12h, after the TLC detection reaction was completed, add Adjust the pH to alkaline (8-10) with NaOH (5mol / L), add the organic solvent dichloromethane (500mL) for extraction, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, and recover the solvent by distillation under reduced pressure. Distilled under reduced...
Embodiment 2
[0049] Add N-pivaloylphenylhydroxylamine (19.3g, 0.1mol), KF (2.9g, 0.05mol) and dichloromethane (200mL) into a stirred 500mL reaction flask in turn, cool to 0°C, and pass through Acyl fluoride (20g, 0.2mol), sealed system, slowly warming up to room temperature, stirring and reacting for 12h, adding saturated brine (50mL) to wash after the reaction, concentrating the organic phase under reduced pressure and distilling dichloromethane, the crude product was subjected to vacuum distillation , to obtain N-pivaloyl 4-fluoroaniline (18.5g), N-pivaloyl 4-fluoroaniline was added to 2mol / L hydrochloric acid (100mL), heated to reflux for 12h, after the reaction was detected by TLC, added Adjust the pH to alkaline (8-10) with NaOH (5mol / L), add the organic solvent dichloromethane (100mL) for extraction, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, recover the solvent by distillation under reduced pressure, and carry out the crude product Distillation u...
Embodiment 3
[0051] Add N-Boc-phenylhydroxylamine (2.09g, 0.01mol), KF (0.29g, 0.005mol) and dichloromethane (20mL) into a stirred 50mL reaction flask in turn, cool to 0°C, and pass through sulfonyl Fluorine (2g, 0.02mol), sealed system, slowly warming up to room temperature, stirring and reacting for 12h, adding saturated brine (25mL) to wash after the reaction, concentrating the organic phase under reduced pressure and distilling dichloromethane, the crude product was subjected to vacuum distillation, To obtain N-Boc-4-fluoroaniline (2.05g), N-Boc-4-fluoroaniline was added to 2mol / L hydrochloric acid (100mL), and reacted at room temperature for 2h. After the reaction was detected by TLC, NaOH (5mol / L L) adjust the pH to alkaline (8-10), add an organic solvent dichloromethane (100mL) for extraction, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, recover the solvent by distillation under reduced pressure, and carry out column chromatography on the crude prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com