Conjugate capable of penetrating blood-labyrinth barrier and preparation method thereof
A conjugate and conjugation technology, applied in the field of conjugates that can pass through the blood labyrinth barrier and its preparation, can solve the problems of high risk, surgical preventive administration, and inconvenience of multiple medications, and achieve the goal of treating neurological disorders. Effects of Deafness, Good Inner Ear Targeting, and Enrichment Function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Building a database
[0073] 1. Based on the sequence analysis of the kunitz region of the active center, the following central skeleton sequence was found, and the construction of the phage-displayed polypeptide library kun-M was designed and completed.
[0074] Build method:
[0075] Library 1: TFYGGRXKRNNF XXXXX (SEQ ID NO: 1)
[0076] X is a random amino acid.
[0077] Long Primer:
[0078] Library1
[0079] 5'-cccaggtgcag ctgcag ACCTTTTATGGTGGTCGTNNKAAACGTAATAATTTTTNNKNNKNNKNNKNNK tctaga ggggacccaggtc-3' (SEQ ID NO: 2)
[0080] Said N is A, T, C or G; said K is G or T
[0081] The restriction sites are underlined, and the uppercase letters are the nucleic acid sequences encoding the peptide library.
[0082] Design the first pair of primers NAG-F: GCCCAGGTGCAGCTG (Tm 57.24) (SEQ ID NO: 3)
[0083] The second pair of primers NAG-R: GACCTGGGTCCCCTTAG (Tm 57.29) (SEQ ID NO: 4)
[0084] The primers were synthesized by Primer Synthesis Company.
...
Embodiment 3
[0124] Example 3 In vivo verification in animals
[0125] 1. Peptide synthesis and fluorescent labeling
[0126] Select M1 for in vivo verification in animals, and send the peptide sequence TFYGGRPKRNNFLRGIR (MW: 2053) to Peptide Synthesis Company for synthetic peptide synthesis and labeled fluorescent purification. A polypeptide with a purity of more than 95% is obtained.
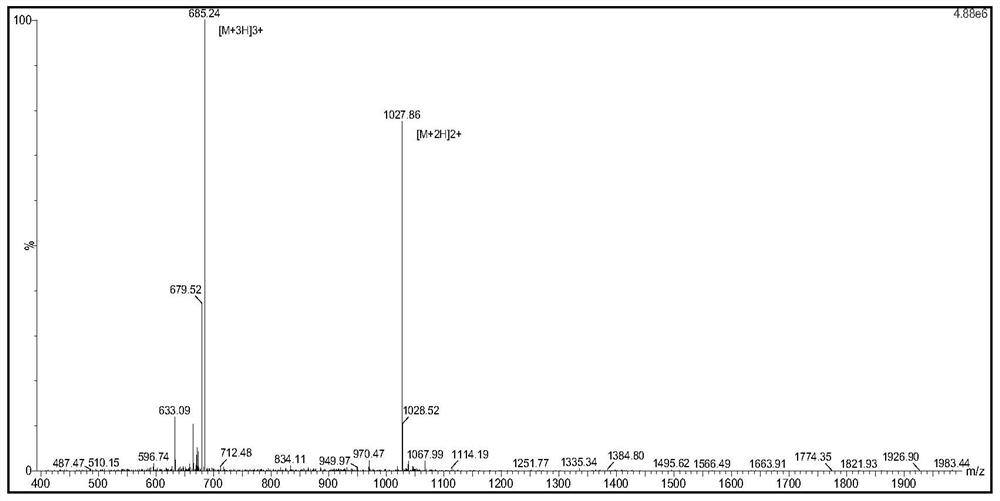
[0127] The detection results of unlabeled peptide mass spectrometry are as follows: image 3 shown. The molecular weight of the peptide is 1027.86*2-2=2053.6, which is consistent with the molecular weight.
[0128] The mass spectrometry detection results of the labeled CY5.5 fluorescent peptide are as follows: Figure 4 Show. The fluorescent molecular weight on the CY5.5 marker is 873.22*3-3-2053=564, which is the molecular weight after the reaction of the cy5.5 fluorescent reagent. It shows that the M1 short body is connected with a fluorescent molecule.
[0129] 2. In vivo detection of fluorescent...
Embodiment 4
[0133] Embodiment 4 prepares curcumin-glutaric acid-NHS active ester
[0134]
[0135] Using glutaric acid as a linker, the phenolic hydroxyl group of glutaric anhydride and curcumin (Curcumin) is condensed to form an ester. Figure 8 shown. Then DCC (dicyclohexylcarbodiimide) is used to activate the carboxyl group, and the NHS (N-hydroxysuccinimide) active ester is coupled with the carboxyl group to obtain curcumin-glutaric acid-NHS active ester, which can be directly combined with protein / The lysine residues of the short peptides are linked. Confirmed by mass spectrometry, curcumin-glutaric acid-NHS active ester product was obtained. Theoretical molecular weight: 579, mass spectrum peak 1: 580 (M+H + ), mass spectrum peak 2: 602M+Na + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com