Method for synthesizing novel glutaric acid compound from bisaryl-substituted non-activated olefin
A technology for activating alkenes and glutaric acid, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of cyanide reactions. Wide application range and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
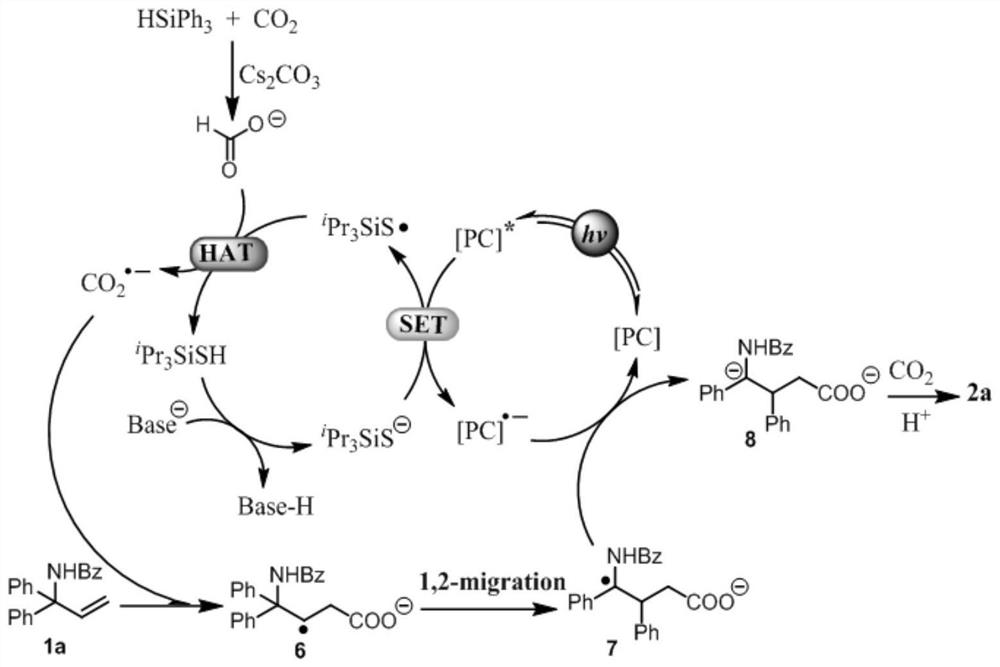
[0028] This example provides a method for constructing novel glutaric acid compounds through the aryl migration strategy of non-activated olefin compounds substituted with different amino groups. The specific steps are as follows:
[0029] In a dried 10mL Schlenk tube (with stirring bar), accurately weigh different amino-substituted non-activated olefin compounds (0.2mmol, 1.0equiv), reducing agent triphenylsilane (104mg, 0.4mmol, 2.0equiv), The photosensitizer 3DPA2FBN (2.6mg, 2mol%) was added to the reaction tube, and then the reaction tube was moved to the glove box to add the base Cs 2 CO 3 (196 mg, 0.6 mmol, 3.0 equiv), after which the reaction tube was sealed using the corresponding stopcock of the reaction tube and removed from the glove box. Under the double-row gas guide system, the gas in the reaction tube is replaced by CO 2 Atmosphere, repeated 3 times; followed by CO 2 Add the hydrogen-absorbing reagent triisopropylsilylthiol (9μL, 0.04mmol, 20mol%) and the ult...
Embodiment 2
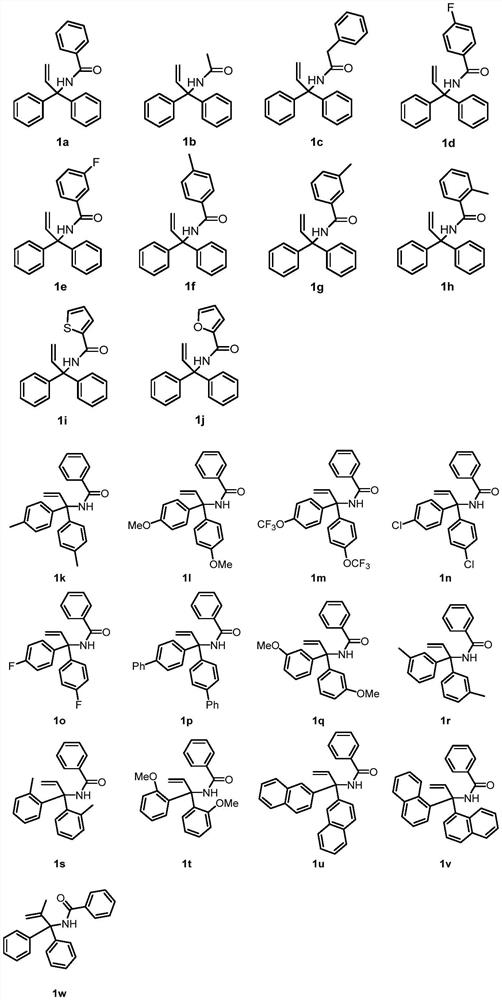
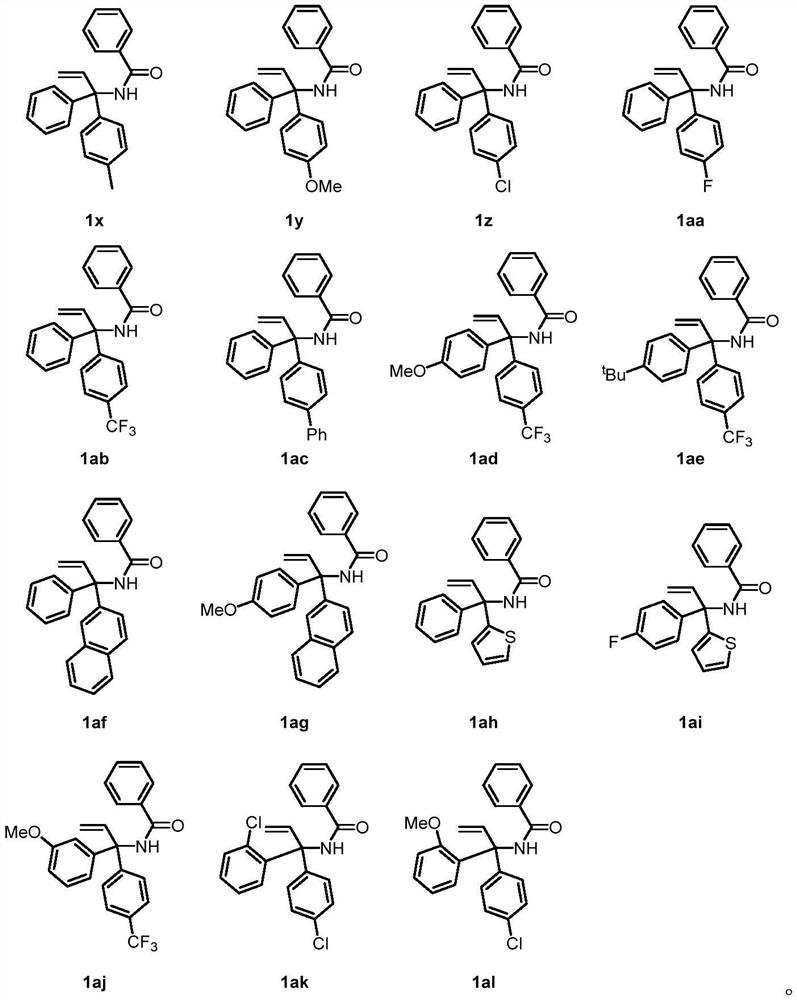
[0034] This example provides a method for constructing a novel glutaric acid compound from a non-activated olefin compound substituted with a symmetrical bisaryl group. The reaction steps are the same as those in Example 1, except that the reaction substrate is different, the specific structure and yield of the product And the ratio of diastereomers is shown in Table 2, and the specific reaction formula is shown in the following formula.
[0035]
[0036] Table 2 Corresponding products, yields and ratios of diastereomers of non-activated olefins substituted with different symmetrical bisaryl groups
[0037]
Embodiment 3
[0039] This example provides a method for constructing novel glutaric acid compounds from non-activated olefin compounds substituted by asymmetric bisaryl groups. The reaction steps are the same as those in Example 1, except that the reaction substrates are different. Ratio and diastereomer ratio are shown in Table 3, and the specific reaction formula is shown in the following formula.
[0040]
[0041] Table 3 Corresponding products, yields and ratios of diastereomers of different asymmetric bisaryl substituted non-activated alkenes
[0042]
[0043] The above experimental results show that non-activated alkene substrates with different amine substitutions, symmetrical aryl substitutions, and asymmetric aryl substitutions can all be synthesized with high yields and regioselectivities as well as moderate to good diastereoselectivities. A novel glutaric acid compound was obtained. Under this reaction system, substrates with different electrical properties can be compatib...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap