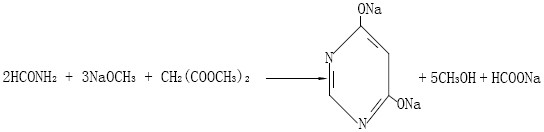
A kind of production method of 4,6-dihydroxypyrimidine
A dihydroxypyrimidine and production method technology, which is applied in the production field of 4,6-dihydroxypyrimidine, can solve the problems of increasing the consumption of raw material formamide, the inability to further increase the product content, and increasing the difficulty of methanol treatment, so as to achieve the goal of reducing waste water The difficulty of treatment, the effect of solving the large amount of wastewater generated, good economic benefits and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
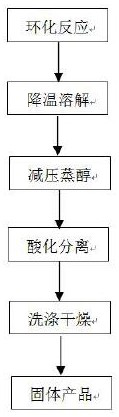
[0042] (1) Cyclization reaction: Add 12600kg of methanol solution containing 30%wt sodium methoxide to a 20000L reactor, then concentrate until the sodium methoxide content is 38%wt, cool down, then add formamide 2172kg, methyl formate 76kg, Close the vent valve of the reactor, heat up to 60°C, then add 2627kg dimethyl malonate, control the reaction temperature at 60-65°C, and the reaction pressure is 0.01MPa, heat preservation and pressure preservation (use the pressure in the reactor after the temperature rise to maintain pressure) to react for 6 hours;
[0043] (2) Cool down and dissolve: cool down to 30°C, add 8930kg of water, stir for 2 hours to dissolve 4,6-dihydroxypyrimidine sodium salt;
[0044] (3) Alcohol distillation under reduced pressure: continuously add the fully dissolved reaction solution into the rectification tower, control the temperature of the tower to 40-50°C, recover anhydrous methanol from the top of the tower, and continuously discharge the 4,6-disti...
Embodiment 2
[0048] (1) Cyclization reaction: Add 12600kg of methanol solution containing 30%wt sodium methoxide to a 20000L reactor, then concentrate until the sodium methoxide content is 40%wt, cool down, then add formamide 2170kg, methyl formate 72kg, Close the vent valve of the reaction kettle, heat up to 65°C, then add 2640kg dimethyl malonate, control the reaction temperature at 65-70°C, and the reaction pressure is 0.03MPa, heat preservation and pressure maintenance (use the pressure in the reaction kettle after the temperature rise to maintain pressure) to react for 5 hours;
[0049] (2) Cool down and dissolve: cool down to 32°C, add 8980kg of water, stir for 1 hour to dissolve 4,6-dihydroxypyrimidine sodium salt;
[0050] (3) Alcohol distillation under reduced pressure: continuously add the fully dissolved reaction solution into the rectification tower, control the temperature of the tower kettle at 50-60°C, recover anhydrous methanol from the top of the tower, and continuously di...
Embodiment 3
[0054](1) Cyclization reaction: Add 12600kg of methanol solution containing 30%wt sodium methoxide to a 20000L reactor, then concentrate until the sodium methoxide content is 36%wt, cool down, then add formamide 2170kg, methyl formate 68kg, Maintain the normal pressure of the reactor, raise the temperature to 70°C, and then add 2635kg of dimethyl malonate, control the reaction temperature at 70-75°C, and the reaction pressure is 0.3MPa (filled with dry nitrogen to maintain the pressure), and keep the temperature for 4 hours;
[0055] (2) Cool down and dissolve: cool down to 35°C, add 8960kg of water, stir for 1 hour to dissolve 4,6-dihydroxypyrimidine sodium salt;
[0056] (3) Alcohol distillation under reduced pressure: continuously add the fully dissolved reaction solution into the rectification tower, control the temperature of the tower kettle at 60-70°C, recover anhydrous methanol from the top of the tower, and continuously discharge the 4,6-distillate from the tower kettl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com