Glycosyl polyether compound liposome as well as preparation method and medicine thereof
A polyether compound and liposome technology, which is applied in liposome delivery, drug combination, nano-drugs, etc., can solve the problems of low bioavailability, affecting drug delivery effect, and short drug delivery time, so as to ensure physiological efficacy , small particle size and narrow particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
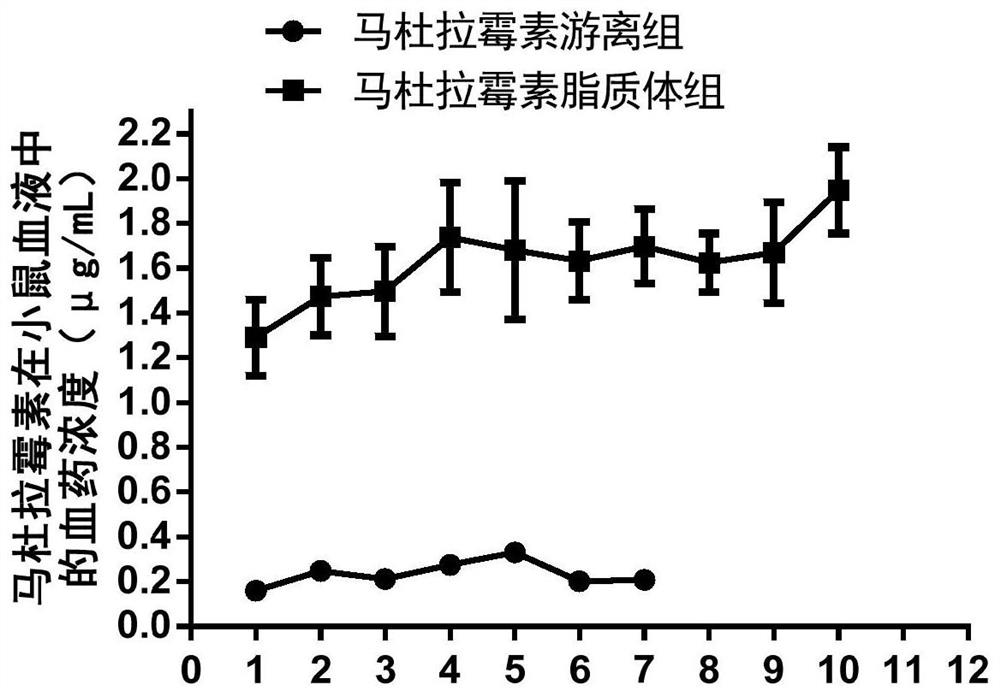
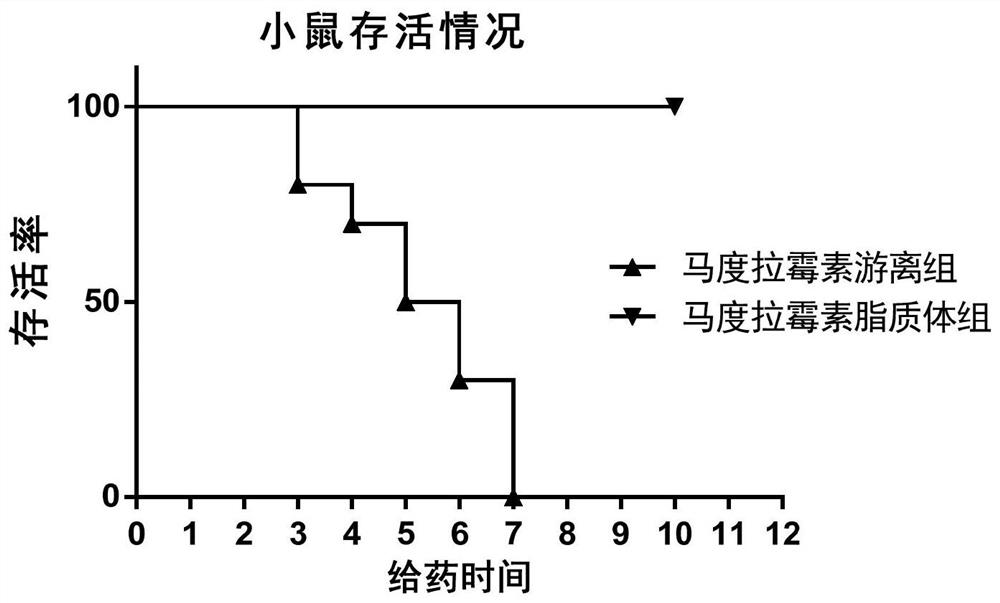
[0052] Embodiment 1 liposome encapsulated maduramycin (also known as maduramycin liposome)
[0053] Example 1 uses maduramycin as a representative compound of the compound shown in formula I for liposome encapsulation. Wherein the specific structural formula of maduramycin is as follows:
[0054]
[0055] Concrete preparation steps are as follows:
[0056] (1) Take by weighing 20g soybean lecithin, 10g cholesterol and 5g maduramycin, and place them in a round bottom bottle;
[0057] (2) Dissolve the mixed solvent of 15mL of chloroform and 2mL of methanol;
[0058] (3) the dissolved solution is placed in a rotary evaporator at normal temperature, and most of the organic solvent is removed by distillation under reduced pressure, and then the residual organic solvent is removed with a nitrogen blower;
[0059] (4) After removing the organic solvent, add a certain amount of sucrose buffer (2125mg sucrose, 94mg glycine and 7mg calcium chloride dihydrate can be mixed and disso...
Embodiment 2
[0060] Example 2 Liposome encapsulation J1-001-1 (also known as J1-001-1 liposome)
[0061] Example 2 uses J1-001-1 as a representative compound of the compound represented by formula II for liposome encapsulation. The specific structural formula of J1-001-1 is as follows:
[0062] (J1-001-1),
[0063] Concrete preparation steps are as follows:
[0064] (1) Weigh 25g of gangliosides, 10g of cholesterol and 5g of J1-001-1, and place them in a round bottom bottle;
[0065] (2) Dissolve the mixed solvent of 15mL of chloroform and 2mL of methanol;
[0066] (3) After the solution is dissolved, it is placed in a rotary evaporator at normal temperature, and most of the organic solvent is removed by distillation under reduced pressure, and then the residual organic solvent is removed with a nitrogen blower;
[0067] (4) After removing the organic solvent, add a certain amount of sucrose buffer solution, ultrasonicate for 20 minutes in a water bath at 50°C, place in a high-pressu...
Embodiment 3
[0068] Example 3 Liposome encapsulation J1-001-2 (also known as J1-001-2 liposome)
[0069] Example 3 uses J1-001-2 as a representative compound of the compound represented by formula II for liposome encapsulation. Wherein the concrete structure of this compound is as follows:
[0070] (J1-001-2),
[0071] Concrete preparation steps are as follows:
[0072] (1) Weigh 25g of gangliosides, 10g of cholesterol and 5g of J1-001-2, and place them in a round bottom bottle;
[0073] (2) Dissolve the mixed solvent of 15mL of chloroform and 2mL of methanol;
[0074] (3) After the solution is dissolved, it is placed in a rotary evaporator at normal temperature, and most of the organic solvent is removed by distillation under reduced pressure, and then the residual organic solvent is removed with a nitrogen blower;
[0075] (4) After removing the organic solvent, add a certain amount of phosphate buffer, ultrasonicate for 20 minutes in a water bath at 50°C, place in a high-pressure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com