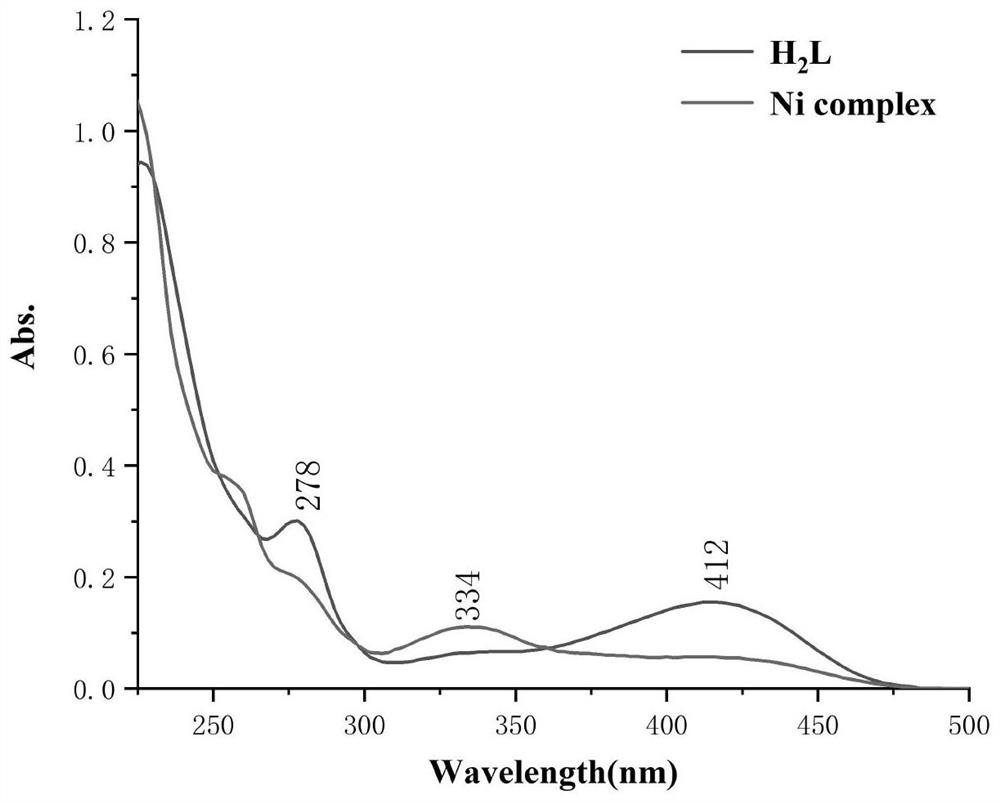
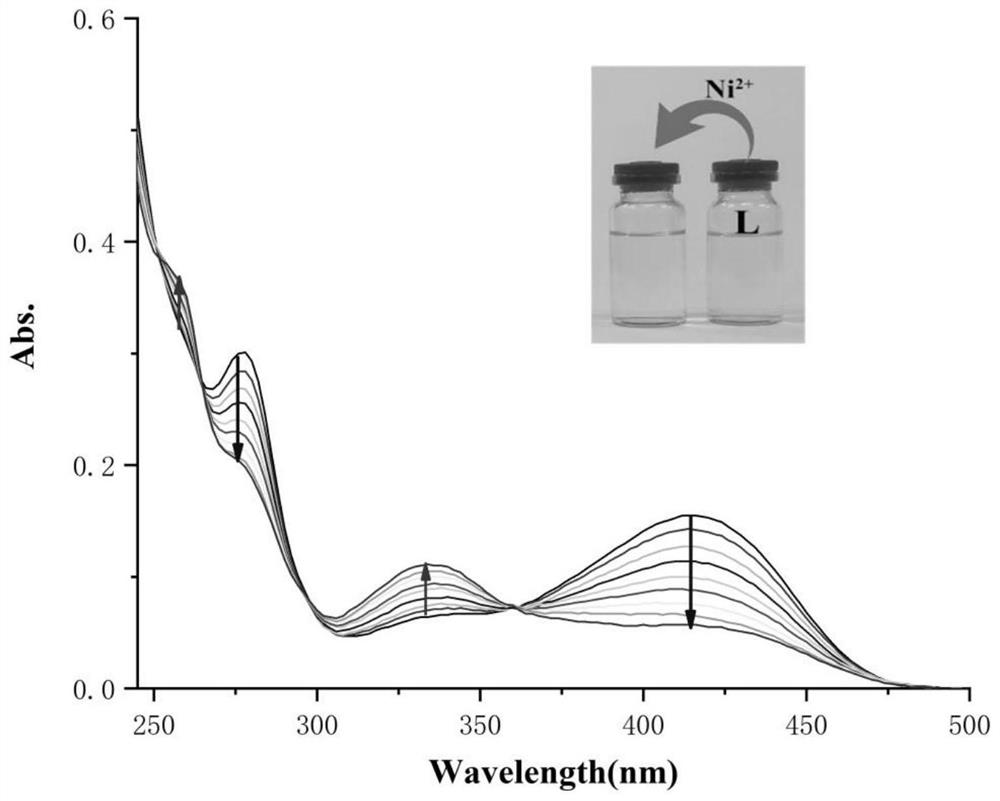
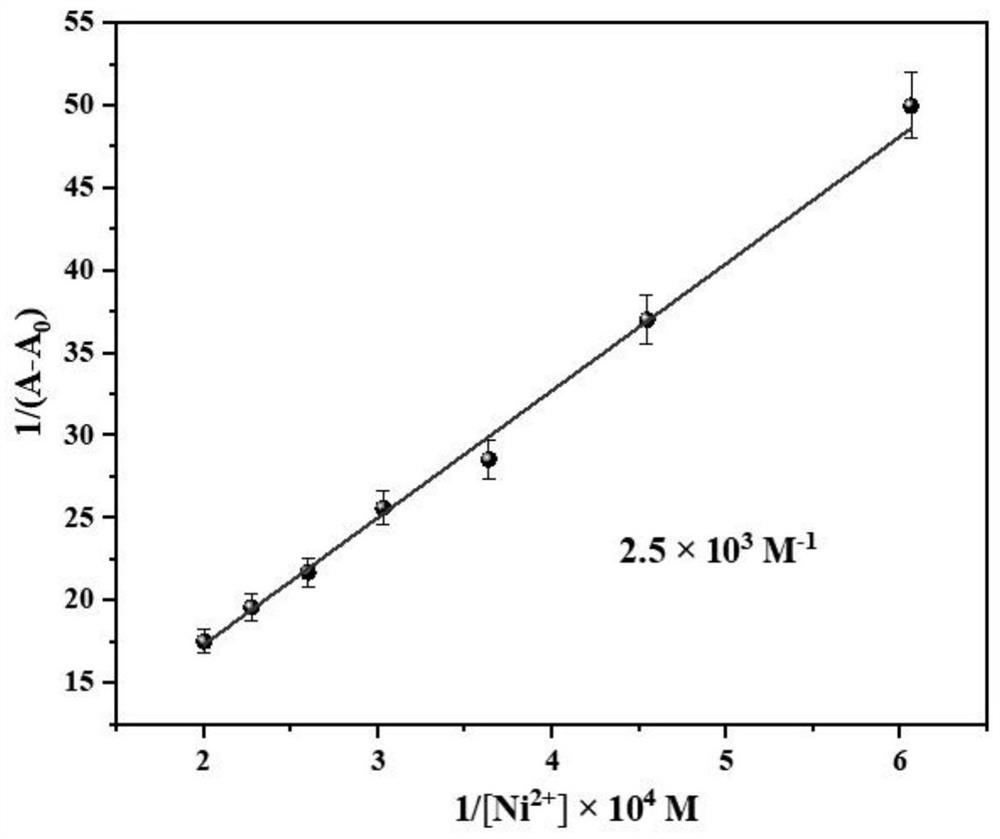
Saldmpn type nickel halide (II) complex as well as preparation method and application thereof
A technology of complexes and nickel halides, which is applied in organic chemical methods, iron-organic compounds, chemical instruments and methods, etc., can solve problems such as weak halogen bond interactions, achieve enhanced fluorescence intensity, simple preparation methods, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of Saldmpn type nickel halide (II) complex of the present invention comprises the steps:
[0039] 1) Dissolve 3,5-dichlorosalicylaldehyde and N, N'-bis(3-aminopropyl)methylamine in methanol and stir for 1.5-2.5 hours;
[0040] 2) Add nickel (II) nitrate and stir for 1-2 hours, then filter to obtain the filtrate;
[0041] 3) Slowly volatilize the filtrate at room temperature to obtain massive brown crystals, wash the crystals several times with polar solvents and filter, and finally dry to obtain the target complex.
[0042] The molar ratio of 3,5-dichlorosalicylaldehyde, N, N'-bis(3-aminopropyl)methylamine to nickel(II) nitrate is 2:1-1.1:1.4-1.5.
[0043] In the step 1, the volume ratio of N, N'-bis(3-aminopropyl)methylamine to methanol is 1:4000-5000.
[0044] In the step 3, the polar solvent is methanol.
[0045] In said step 3, the volatilization time is 7 days.
[0046] The complex compound of the present invention is used as a logic gate...
Embodiment 1
[0052] Take a mixture of 0.019g (0.1 mmol) 3,5-dichlorosalicylaldehyde and 8.1ul (0.05 mmol) N, N'-bis(3-aminopropyl)methylamine in 20 mL of methanol and stir at room temperature for 2 hours, Then 0.022 g (0.072 mmol) of nickel(II) nitrate were added, the resulting light brown mixture was further stirred for 1.5 hours and filtered to obtain a filtrate. The resulting filtrate was slowly evaporated over 7 days to give blocky brown crystals. Filter the blocky brown crystals, wash with methanol for several times, and then open and dry at room temperature to obtain {[Ni II (3,5-Cl-saldmpn)] 3} (1), the yield is 36.5%.
Embodiment 2
[0054] Take a mixture of 0.010g (0.05 mmol) 3,5-dichlorosalicylaldehyde and 4 ul (0.025 mmol) N, N'-bis(3-aminopropyl)methylamine in 20 mL of methanol and stir at room temperature for 1.5 hours, Then 0.011 g (0.036 mmol) nickel(II) nitrate was added, the resulting light brown mixture was further stirred for 1 hour and filtered to obtain a filtrate. The resulting filtrate was slowly evaporated over 6 days to give blocky brown crystals. Filter the blocky brown crystals, wash with methanol for several times, and then open and dry at room temperature to obtain {[Ni II (3,5-Cl-saldmpn)] 3} (1), the yield is 34%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com