Monomethyl fumarate-carrier conjugates and methods of their use
A technology of monomethyl fumarate and conjugates, which can be used in drug combinations, pharmaceutical formulations, active ingredients of esters, etc., and can solve the problem of underutilization of small molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0377] Preparation of conjugates
[0378] Compounds can be prepared using synthetic methods and reaction conditions known in the art. Optimum reaction conditions and reaction times may vary with the reactants used. Solvents, temperatures, pressures and other reaction conditions can be selected by one of ordinary skill in the art unless otherwise indicated.
[0379] Glycoside Preparation Strategy #1: (Substitution)
[0380] plan 1
[0381]
[0382] In Scheme 1, a polyacylated sugar, compound 1, is treated with monomethyl fumarate compound 2 in a suitable solvent, optionally in the presence of a catalyst, wherein n represents an integer from 1 to 3, m Represents an integer from 0 to 1, and R is equal to C1-10 alkyl. Suitable catalysts include pyridine, dimethylaminopyridine, trimethylamine, and the like. The catalyst may be used in an amount ranging from 0.01 to 1.1 equivalents relative to Compound 2. Suitable solvents include dichloromethane, ethyl acetate, diethyl eth...
Embodiment 1
[0468] Example 1: Preparation of Exemplary Conjugates of the Invention
[0469]
[0470] Compound 1: Fumaric acid ((2S,3S,4R,5R,6S)-6-methyl-3,4,5-tris(propionyloxy)tetrahydro-2H-pyran-2-yl)methyl base ester
[0471] at 20°C in N 2 Downward [(2S,3R,4R,5S)-6-hydroxy-2-methyl-4,5-di(propionyloxy)tetrahydropyran-3-yl]propionate (0.5g, 1.50 mmol, 1 equiv) and (E)-4-methoxy-4-oxo-but-2-enoic acid (234.87 mg, 1.81 mmol, 1.2 equiv) in THF (5 mL) were added in one portion DCC (620.82 mg, 3.01 mmol, 2 equiv) and DMAP (91.90 mg, 752.23 μmol, 0.5 equiv) in . The mixture was stirred at 20 °C for 12 h. LC-MS indicated complete consumption of [(2S,3R,4R,5S)-6-hydroxy-2-methyl-4,5-bis(propionyloxy)tetrahydropyran-3-yl]propionate , and a main peak with the expected m / z was detected. The reaction mixture was filtered and concentrated under reduced pressure to yield a residue. The residue was purified by preparative HPLC (water + 0.04% (v / v) HCl / MeOH) and fumaric acid ((2S,3S,4R,5R,6S...
Embodiment 2
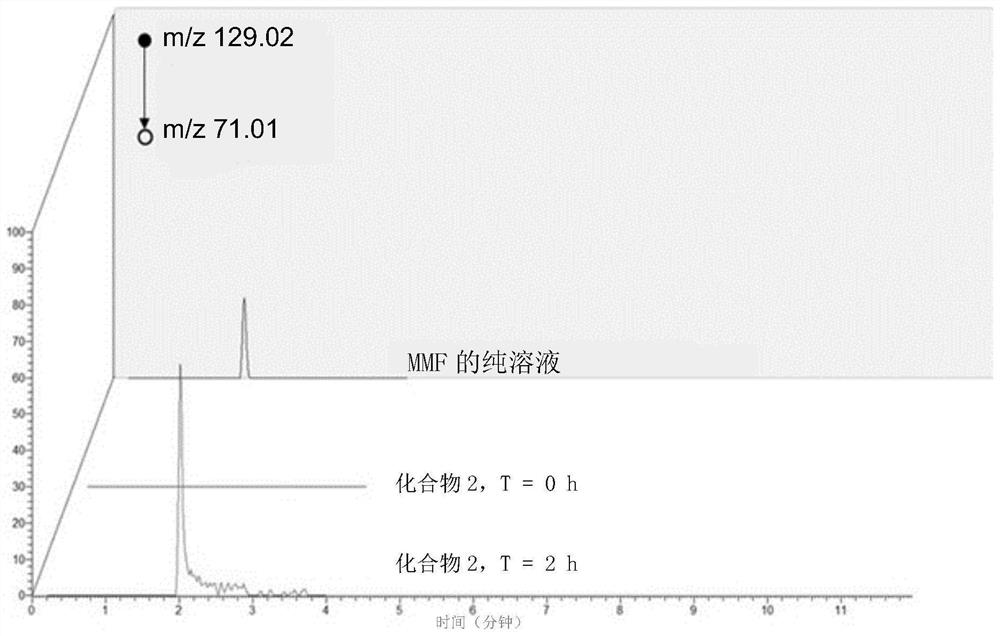
[0708] Example 2: In vitro DMPK degradation assay
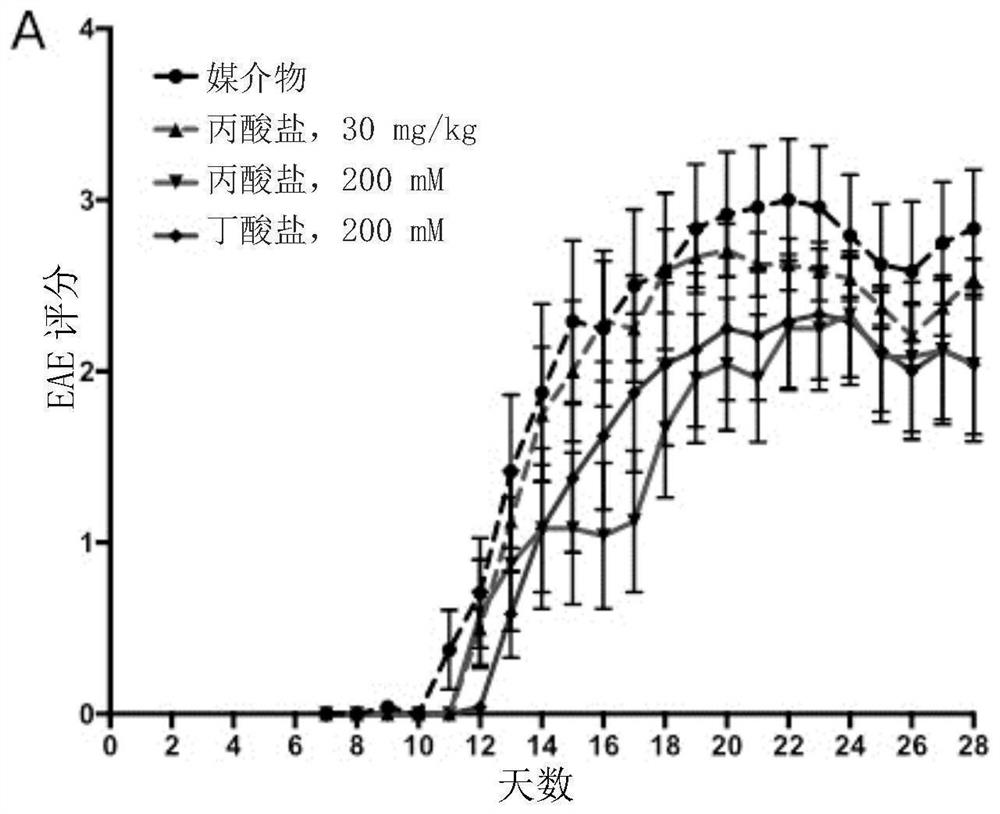
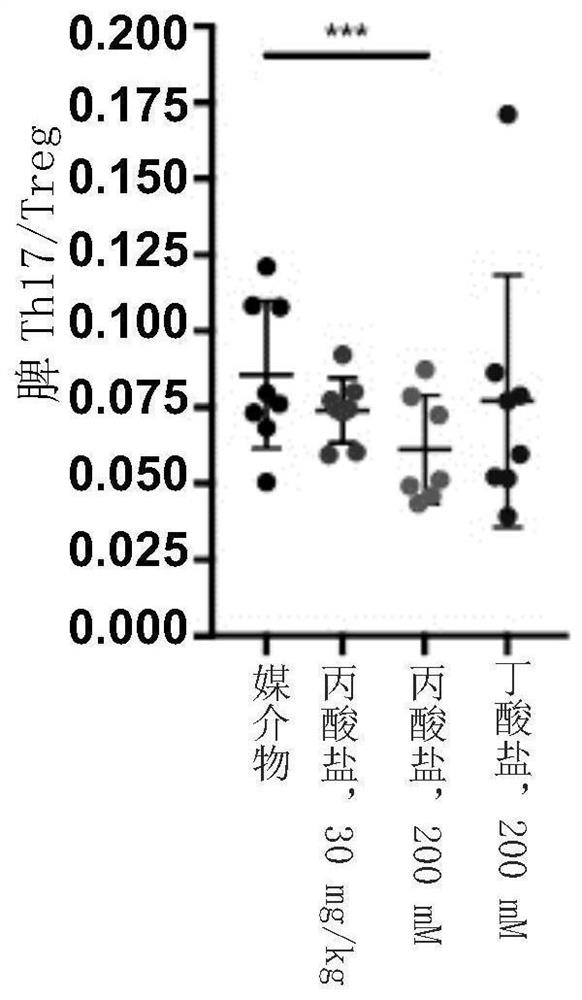
[0709] The conjugates disclosed herein may be stable at a range of physiological pH levels and may be activated by enzymes present in the local microenvironment at the desired site of action (e.g., in the gastrointestinal tract, such as in the stomach, small intestine, or large intestine). ) are selectively cut. Conjugates were tested for chemical stability over a range of pH levels as well as their ability to degrade in representative in vitro systems. Data for selected conjugates are shown below.
[0710] Assay 1. Stability of the conjugates in simulated gastric fluid (SGF). This assay was used to assess the stability of the conjugates in the stomach.
[0711] By dissolving 2 g of sodium chloride in 0.6 L of ultrapure water ( Millipore Sigma, Darmstadt, Germany) to prepare media. The pH was adjusted to 1.6 with 1N hydrochloric acid, and then the volume was adjusted to 1 L with purified water.
[0712] 60mg FaSSIF pow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com