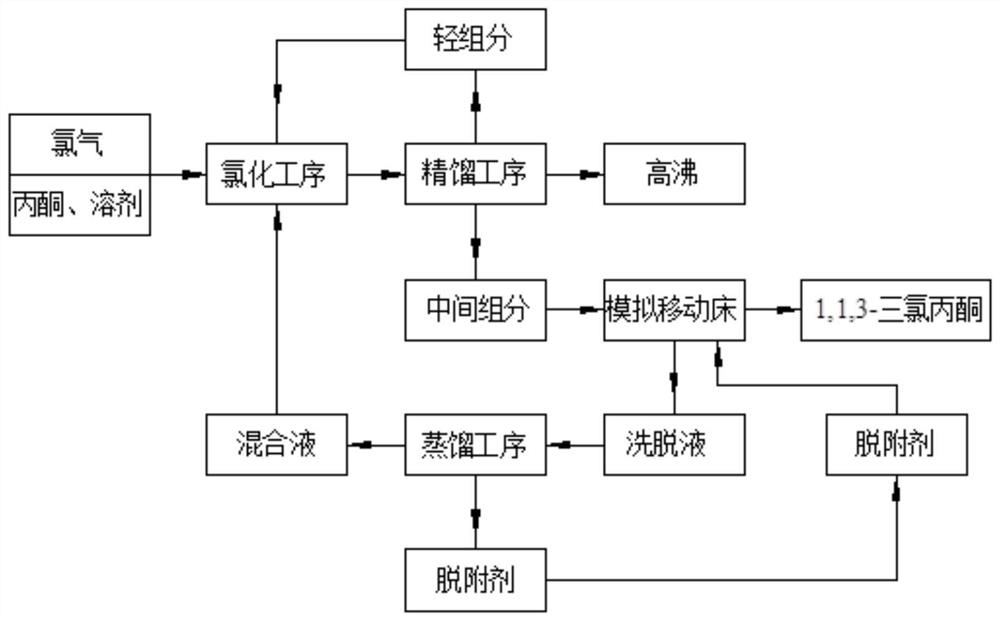
Production method of 1,1,3-trichloroacetone
A technology of trichloroacetone and dichloroacetone, applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carbonyl compounds, etc., can solve the problems of high folic acid production cost, complicated process, and low product purity, and achieve Improving product competitiveness, avoiding separation operations, and making the process green and environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Add the solvent chloroform and acetone into a 5L photochlorination circulation reactor in a mass ratio of 1:1.02, control the liquid level in the reactor at 65%, and preheat the material to 44.8°C under stirring ;
[0040] (2) Turn on the 457nm blue-green light source (rated power 50W), turn on the chlorine gas into the equipment, and gradually adjust the flow of chlorine gas to 0-60L / h to generate a reaction liquid, and control the chlorination reaction by adjusting the flow of cooling water The temperature is -10°C;
[0041] (3) In the reaction process, take the reaction solution in the step (2) every 15min to remove the solvent chloroform as the detection solution, and use the gas chromatography area normalization method to analyze the detection solution composition. After the solvent peak is deducted from the detection solution chromatogram The content of 1,1,3-trichloroacetone in the spectrum reaches 34.5%, the chlorine flow is stopped, and the reaction is con...
Embodiment 2
[0046] (1) Add the solvent chloroform and acetone into a 5L photochlorination circulation reactor in a mass ratio of 1:1.98, control the liquid level in the reactor at 70%, and preheat the material to 44.9°C under stirring ;
[0047] (2) Turn on the 403nm purple light source (rated power 50W), turn on the chlorine gas feeding equipment, and gradually adjust the flow rate of chlorine gas to 0-80L / h to generate a reaction liquid, and at the same time control the chlorination reaction at 15.5 by adjusting the cooling water flow rate ℃;
[0048] (3) In the reaction process, take the reaction solution in the step (2) every 15min to remove the solvent chloroform as the detection solution, and use the gas chromatography area normalization method to analyze the detection solution composition. After the solvent peak is deducted from the detection solution chromatogram The content of 1,1,3-trichloroacetone in the spectrum reaches 33.9%, stop the chlorine flow, and continue the reaction...
Embodiment 3
[0053] (1) Add the solvent chloroform and acetone into a 5L photochlorination circulation reactor in a mass ratio of 1:2.99, control the liquid level in the reactor at 80%, and preheat the material to 44.7°C under stirring ;
[0054] (2) Turn on the 354nm purple light source (rated power 50W), turn on the chlorine gas feeding equipment, and gradually adjust the flow rate of chlorine gas to 0-90L / h to generate a reaction liquid, and at the same time control the chlorination reaction at 43.6 by adjusting the cooling water flow rate ℃;
[0055] (3) In the reaction process, take the reaction solution in the step (2) every 15min to remove the solvent chloroform as the detection solution, and use the gas chromatography area normalization method to analyze the detection solution composition. After the solvent peak is deducted from the detection solution chromatogram The content of 1,1,3-trichloroacetone in the spectrum reaches 33.5%, the chlorine flow is stopped, and after the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com