Production and purification method of teriparatide hPTH (1-34)
A technology of teriparatide and purification method, which is applied in the field of genetic engineering, can solve the problems of high price of teriparatide products, cumbersome purification steps, and low yield, and achieve fewer steps, high yield and purity, and low equipment requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
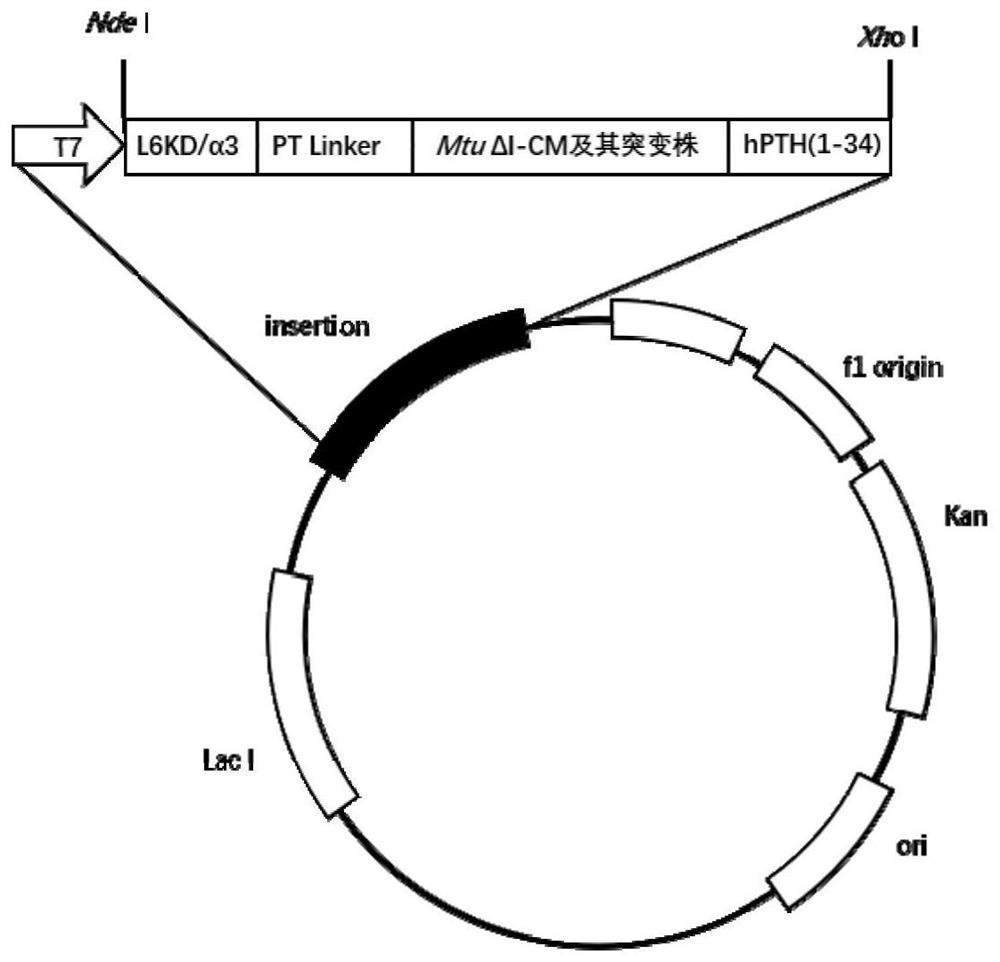
[0060] Example 1: Construction of Teriparatide Fusion Protein Expression Constructs Containing Different Intein MtuΔI-CM Mutants The expression vector used in the examples of this application is a fusion protein expression vector containing 4 different MtuΔI-CM mutants (pET30a-L6KD-PT-MtuΔI-CM-hPTH(1-34), pET30a-L6KD-PT-MtuΔI-CM m1-hPTH(1-34), pET30a-L6KD-PT-MtuΔI-CM m2-hPTH(1 -34), pET30a-L6KD-PT-MtuΔI-CM m3-hPTH(1-34)). Figure 1a Schematic diagram for expression and purification of teriparatide by cSAT method; Figure 1b It is a structural schematic diagram of the cSAT-based teriparatide fusion protein expression vector.
[0061] First construct the pET30a-L6KD-PT-MtuΔI-CM m2-hPTH(1-34) plasmid, the plasmid constructs the required primers, and the oligonucleotides shown in Table 1 are designed by oligo 7 and synthesized by Shanghai Sangong primers. First, the amino acid sequence of teriparatide hPTH (1-34) was obtained from the literature (Teriparatide: A Review). The seq...
Embodiment 2
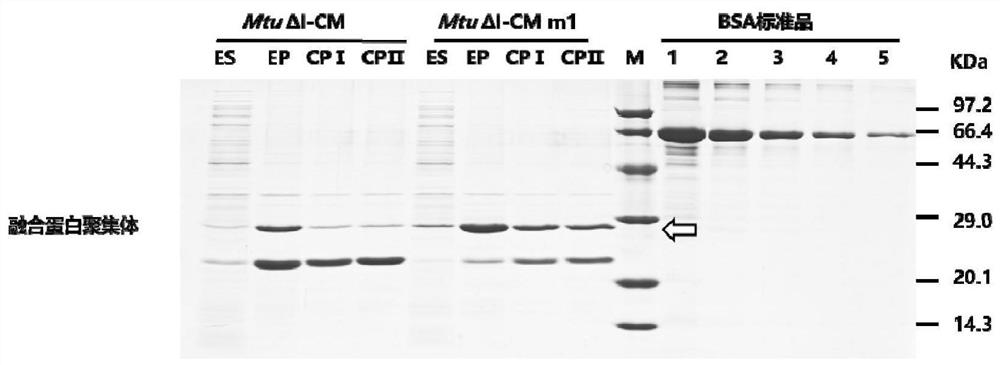
[0067] Example 2: Expression and purification of teriparatide fusion proteins containing different mutant strains of intein MtuΔI-CM
[0068] Containing plasmids (pET30a-L6KD-PT-MtuΔI-CM-hPTH(1-34), pET30a-L6KD-PT-MtuΔI-CM m1-hPTH(1-34), pET30a-L6KD-PT -MtuΔI-CM m2-hPTH(1-34), pET30a-L6KD-PT-MtuΔI-CM m3-hPTH(1-34)) BL21(DE3) strains were inoculated into LB liquid containing 50 μg / mL kanamycin medium, and cultured in a shaker at 37°C until logarithmic phase (OD 600 =0.4~0.6), add final concentration 0.2mMIPTG, culture conditions are: 18°C, 250rpm, harvest cells after 24 hours, and measure bacterial concentration OD 600 (The following will be 1mL of OD 600 The amount of cells equal to 1 is called 1OD).
[0069] The thalline was treated with lysis buffer B1 (Tris of 2.4g, NaCl of 29.22g, NaCl of 0.37g 2 ·EDTA·2H 2 O was dissolved in 800mL water, adjusted to pH 8.5, added water to make up to 1L), resuspended to 20OD / mL, and subjected to ultrasonic crushing (crushing condition...
Embodiment 3
[0077] Example 3: Expression and purification of teriparatide hPTH(1-34) fusion protein using fermentation medium
[0078] Two BL21 (DE3) bacterial strains constructed in Example 1 (respectively containing pET30a-L6KD-PT-MtuΔI-CMm2-hPTH (1-34) plasmid and pET30a-L6KD-PT-MtuΔI-CMm3-hPTH (1 -34) plasmid) was inoculated into a fermentation medium (Shao-Yang Hu et al., 2004) containing 50 μg / mL kanamycin, and cultured in a shaker at 37°C until logarithmic phase (OD 600 = 0.4~0.6), adding a final concentration of 0.2mM IPTG, inducing at 18°C for 24 hours, harvesting the cells, and measuring the bacterial concentration OD 600 . 1 mL of OD 600 A cell mass of 1 is called 1OD. The components of the fermentation medium used are shown in Table 4. Glucose was sterilized separately from other components, sterilized at 121°C for 20 minutes, and the trace element solution was filtered and sterilized on an ultra-clean bench with a 0.22 μm filter head. After the medium was prepared, kan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Expression | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com