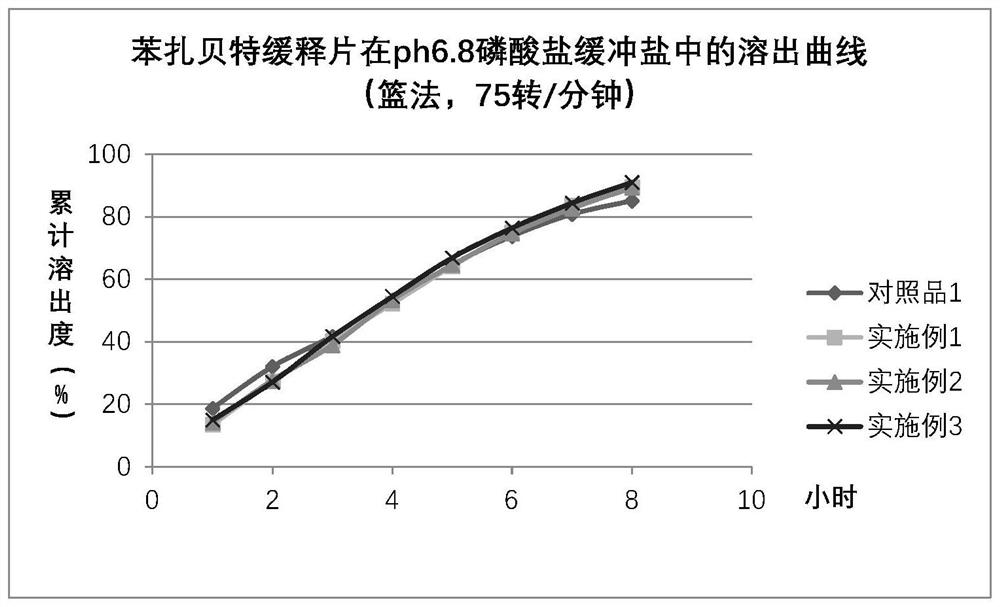
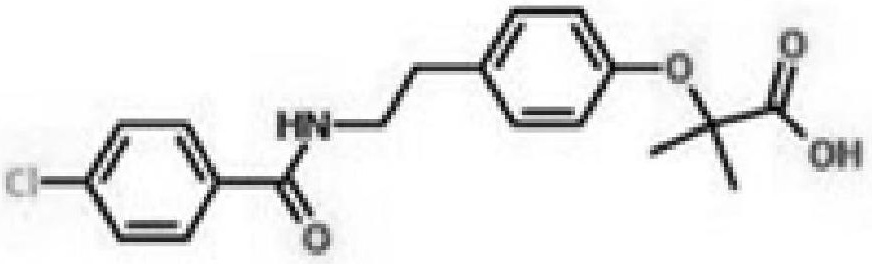
Bezafibrate sustained-release tablet and preparation method thereof
A technology of bezafibrate and sustained-release tablets, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of difficulty in achieving identity, difficulties in sustained-release tablets, and the amount of excipients minor issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1: Bezafibrate sustained-release tablet and preparation method thereof
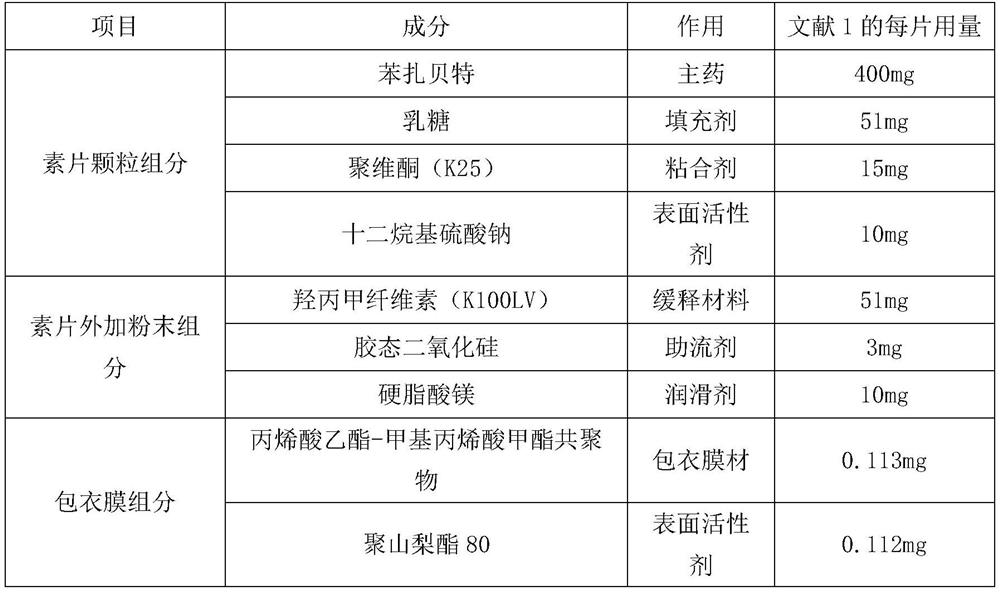
[0091] 1. Composition:
[0092] 1) Tablet core: Weigh 400g of bezafibrate, 31g of 200 mesh lactose, 30g of povidone K25, 15g of sodium lauryl sulfate, 51g of hydroxypropyl methylcellulose K100LV, 3g of colloid, magnesium stearate 10g, spare.
[0093] 2) Coating layer: ethyl acrylate-methyl methacrylate copolymer aqueous dispersion 47.4mg, polysorbate 8014.1mg, hydroxypropyl methylcellulose E5 269.4mg, polyethylene glycol 8000141.7mg, 200 mesh lactose 472.5mg, talc 685.0mg, titanium dioxide 283.5mg, sodium citrate 9.5mg.
[0094] 2. Preparation method:
[0095] 1) Tablet core preparation
[0096] Take lactose, povidone K25, sodium lauryl sulfate, hydroxypropyl methylcellulose K100LV, colloidal silicon dioxide, magnesium stearate respectively according to weight, then pass through 40 mesh sieves respectively, for subsequent use;
[0097] Raw material wetting: take bezafibrate, put it ...
Embodiment 2
[0107] Embodiment 2: Bezafibrate sustained-release tablet and preparation method thereof
[0108] 1. Tablet core: bezafibrate 400g, lactose 29g, povidone K25 28g, sodium lauryl sulfate 12g, hydroxypropyl methylcellulose K100LV 48g, colloidal silicon dioxide 1g, magnesium stearate 8g .
[0109] 2. Coating layer: ethyl acrylate-methyl methacrylate copolymer aqueous dispersion 47.4mg, polysorbate 8014.1mg, hydroxypropyl methylcellulose E5 269.4mg, polyethylene glycol 8000141.7mg, 200 mesh lactose 472.5mg, talcum powder 685.0mg, titanium dioxide 283.5mg, sodium citrate 9.5mg, spare.
[0110] 3. The preparation process is the same as in Example 1.
Embodiment 3
[0111] Embodiment 3: Bezafibrate sustained-release tablet and preparation method thereof
[0112] 1. Tablet core: bezafibrate 400g, lactose 34g, povidone K25 32g, sodium lauryl sulfate 18g, hydroxypropyl methylcellulose K100LV 55g, colloidal silicon dioxide 5g, magnesium stearate 12g .
[0113] 2. Coating layer: ethyl acrylate-methyl methacrylate copolymer aqueous dispersion 47.4mg, polysorbate 8014.1mg, hydroxypropyl methylcellulose E5 269.4mg, polyethylene glycol 8000141.7mg, 200 mesh lactose 472.5mg, talc 685.0mg, titanium dioxide 283.5mg, sodium citrate 9.5mg.
[0114] 3. The preparation process is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com