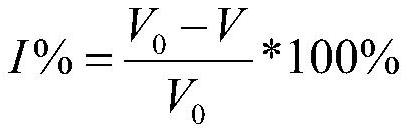Chromone oxadiazole compound as well as preparation method and application thereof
A technology of chromone-linked oxadiazole and compound, which is applied in the field of chromone-linked oxadiazole compound and its preparation, can solve the problems of high toxicity and high cost, and achieve the effect of extensive research prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
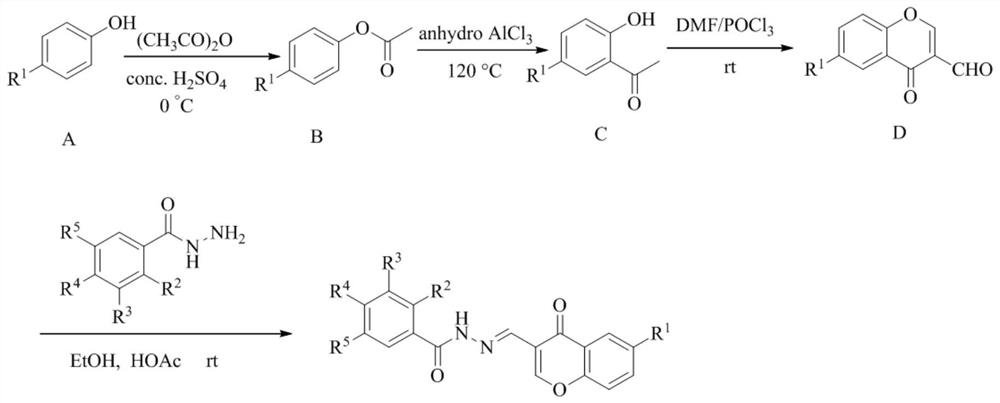
[0023] Pass through figure 1 The steps shown are synthetic intermediate F, i.e. N'-色 ketone-benzoyl hydrazide, and the structural formula is as follows:
[0024] The specific preparation process is: Take 1 mmol of substituted benzoxide hydrazide and 1 mmol 6-substituted-3-formyl ketone mixed in 25 ml of ethanol, add 3 drops of HOAc, and stirred at room temperature for 6 h. There is white turbidity generation, TLC monitors until the reaction is complete, there is a new compound generation. The filtration was filtered, ethanol was washed multiple times until the TLC was removed in the filtrate, and the reactant was reactive. Dry cakes to get pure powder products.
Embodiment 2
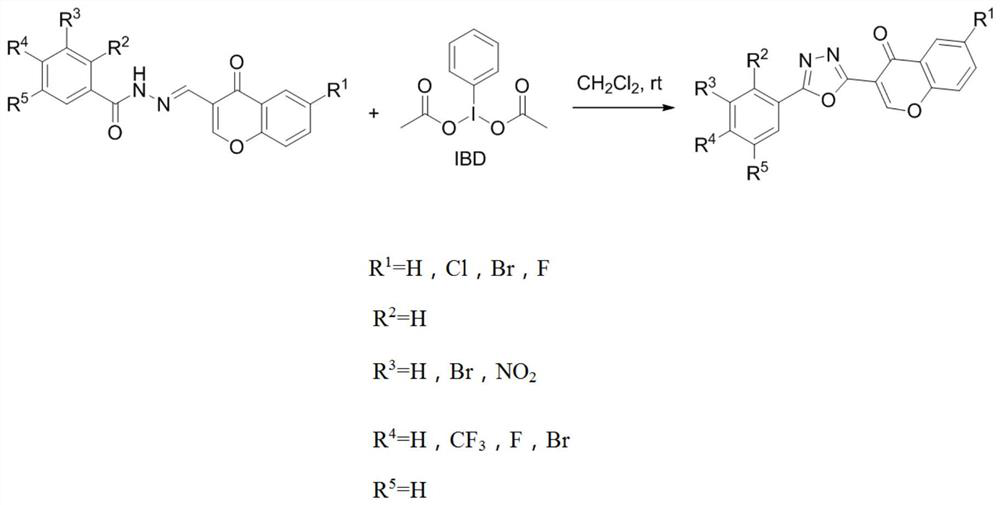
[0026] (1) Compound I-1, i.e. Show:
[0027] Preparation process figure 2 As shown, specifically: 0.9865 g (2.74 mmol) N '- ((4-oxo-4H-ketone-3-) methylene) -4- (trifluoromethyl) benzoylide hydrazide and 1.413 G (3.28mmol, 1.2 times) IBD solid mixed in 20 mlch 2 CL 2 The reaction was stirred at room temperature. After about 10 min, the reaction system gradually became clear. After 1 hours, the reaction system was completely yellow, and TLC monitors showed that the reactants were transformed. Steamed removal CH 2 CL 2 20 ml of EtOAc was added, stirred at room temperature for 10 min, filtration, EtOAc.
[0028] The resulting pure product was white powder, the yield was 70%, the melting point was 236-240 ° C, the molecular formula was c 18 Hide 9 Fly 3 N 2 O 3 The result of the test is 1 H NMR (600MHz, CDCL 3 Δ8.87 (S, 1H, = CH-O), 8.42-7.47 (M, 8H, ARH). MS (EI) M / Z: 358.01 (M) + .Anal.calcd for c 18 Hide 9 Fly 3 N 2 O 3 C, 60.34; H, 2.53; N, 7.82.Found: C, 60.52; H, 2.61; N, 7.6...
Embodiment 3
[0069] The 20 compounds of the above synthesis were carried out by CY-FBP / SBPaser inhibitory experiments. CY-FBP / SBPase can catalyze hydrolyzed sugar-1,6-diphosphate as fructose-6-phosphate (F6P) and inorganic phosphate (Pi). The product Pi can form a blue-green complex with a blue-green complex of the alkaline dye peacock green and ambient ammonium molybdate in the presence of polyol, and can be determined by detecting changes in ultraviolet absorbance values at 620 nm at 620 nm. -Fbp / sbpase enzyme activity, thereby detecting the suppression effect of the above compound on CY-FBP / SBPase.
[0070] The specific experiment process is as follows:
[0071] (1) 50 mM Tris-HCl (pH 8.0), 15mm MgCl in the reaction system 2 , 10mm DTT, suitable concentration of CY-FBP / SBPase and a certain concentration gradient inhibitor;
[0072] (2) Add 0.5 mM substrate (FBP) to start the reaction, and incubated at 37 ° C for 5 min, the catalytic reaction was terminated at 1 M Hybridic acid; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com