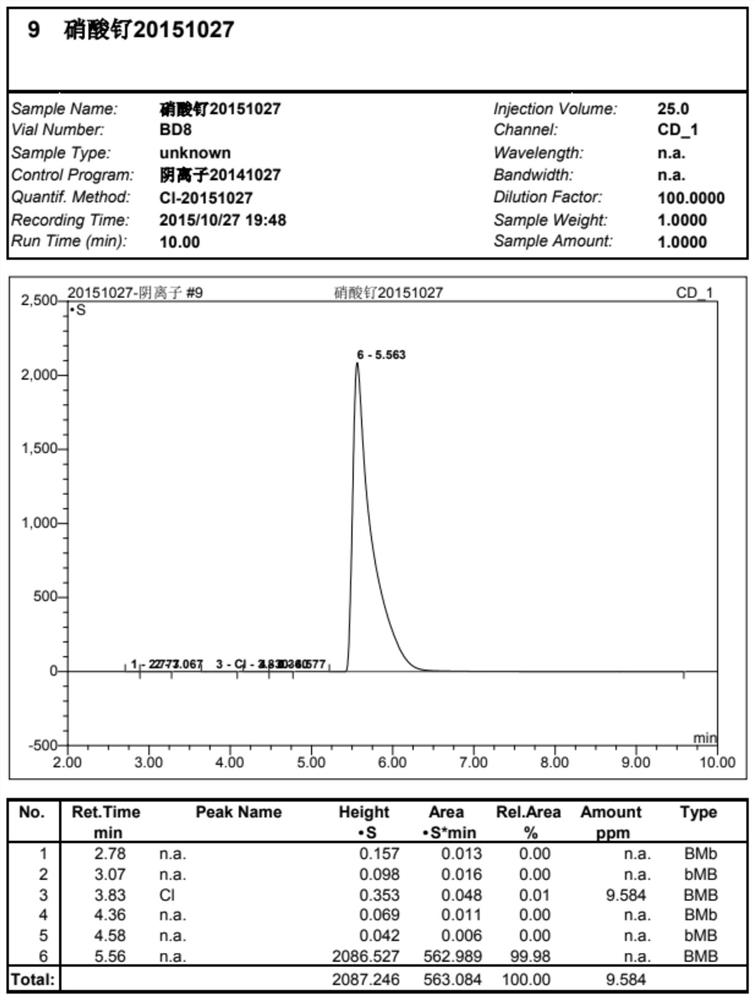
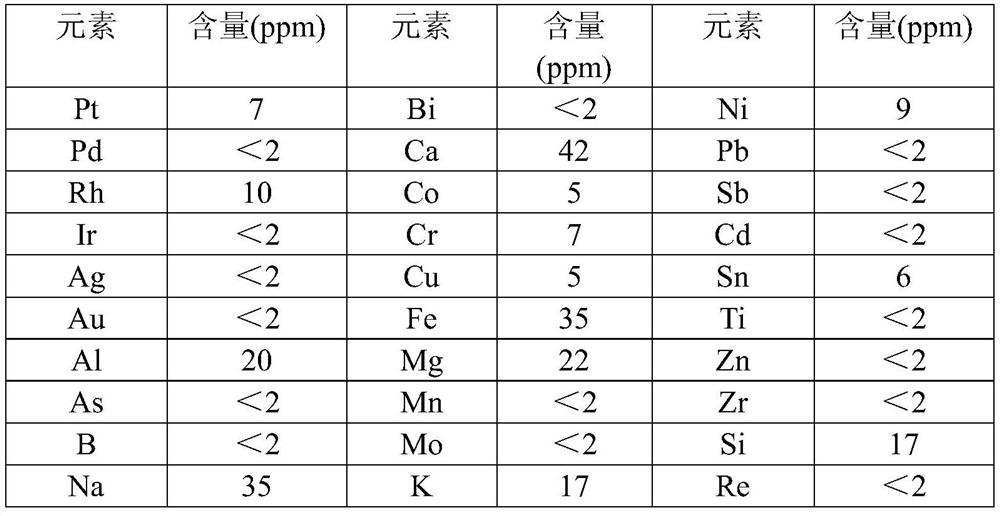
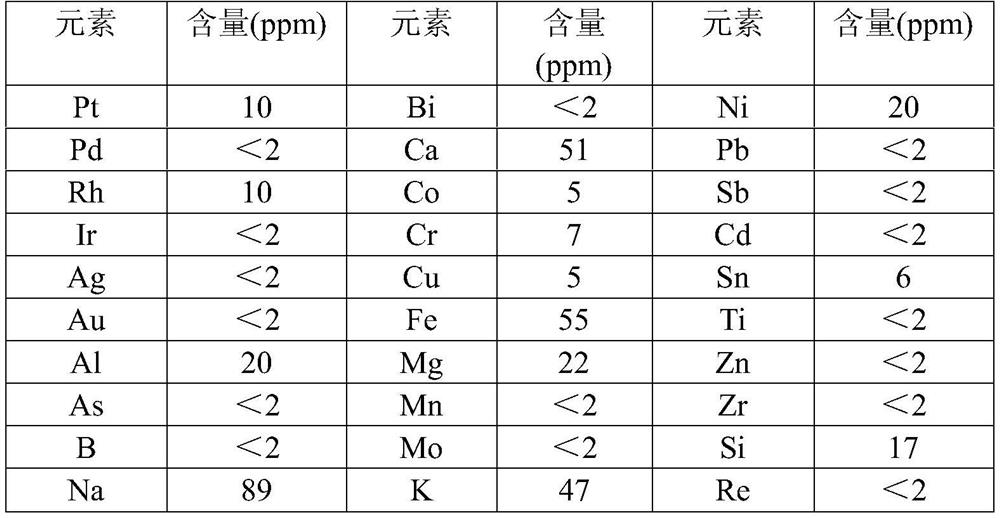
Process method for synthesizing liquid ruthenium nitrate
A synthesis process, the technology of ruthenium nitrate, applied in the field of ruthenium nitrosyl nitrate synthesis, can solve the problems of high content of sodium ion and chloride ion and low concentration of liquid products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) 500 g of ruthenium powder was added to 15 l 15% sodium hydroxide solution, and excess of chlorine gas was introduced to ensure sufficient oxidation, and the reaction temperature was controlled at 85-90 ° C. The liquid level is boiling, there is golden water mist, surrounded with golden yellow bubbles, stopping accession to chlorine;
[0034] 2), adding ethanol reducing agent according to the ratio of the rhodium to 1: 0.6, the color of the solution is black, filtered to obtain Ru 4+ Black precipitate.
[0035] 3), using 0.5% nitric acid and 0.3% ammonia, alternately washed with water, and filtered the filter cake after washing.
[0036] Note: 0.5% nitric acid formulation, nitric acid volume = (filter cake mass × 3 × 0.5%) ÷ (0.65 × 1.41)
[0037] 0.3% ammonia water formulation, ammonia water volume = (filter cake mass × 3 × 0.3% × 4) ÷ 0.9;
[0038] The present formula indicates how to utilize 65% mass concentrations of commercially available nitroths, density of 141 g / ...
Embodiment 2
[0042] 1) 500 g of ruthenium powder was added to 15 l 15% sodium hydroxide solution, and chlorine gas was introduced, and the reaction temperature was controlled at 85-90 ° C. The liquid level is boiling, there is golden water mist, surrounded with golden yellow bubbles, stopping accession to chlorine;
[0043] The ethanol reducing agent is added to 1: 0.7 with the ruthenium ratio, the solution color is black, filtered to obtain Ru 4+ Black precipitate.
[0044] It was washed with 0.5% nitric acid and 0.3% ammonia, and the filter cake was collected after washing.
[0045] Note: 0.5% nitric acid formulation, nitric acid volume = (filter cake mass × 3 × 0.5%) ÷ (0.65 × 1.41)
[0046] 0.3% ammonia water formulation, ammonia volume = (filter cake mass × 3 × 0.3% × 4) ÷ 0.9
[0047] According to the water washing filter, the amount of 5.5 times the mass of the mass of 5.5% is reacted with the filter cake. The reaction temperature was controlled at 85-90 ° C, and the reaction was added ...
Embodiment 3
[0050] 1) 500 g of ruthenium powder was added to 15 l 15% sodium hydroxide solution, and chlorine gas was introduced, and the reaction temperature was controlled at 85-90 ° C. The liquid level is boiling, there is golden water mist, surrounded with golden yellow bubbles, stopping accession to chlorine;
[0051] The ethanol reducing agent is added to 1: 0.8 with the rhodium ratio, and the solution color is black, filtered to obtain Ru. 4+ Black precipitate.
[0052] It was washed with 0.5% nitric acid and 0.3% ammonia, and the filter cake was collected after washing.
[0053] Note: 0.5% nitric acid formulation, nitric acid volume = (filter cake mass × 3 × 0.5%) ÷ (0.65 × 1.41)
[0054] 0.3% ammonia water formulation, ammonia volume = (filter cake mass × 3 × 0.3% × 4) ÷ 0.9
[0055] The 65% nitric acid containing 6 times mass of the ruthenium was carried out in water washing cake. The reaction temperature was controlled at 85-90 ° C, and the reaction was added for about 9 hours, and 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com